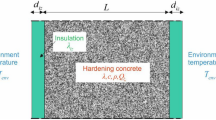
Abstract
Phase-change materials (PCMs) can store/release thermal energy within a small temperature range. This is of interest in various industrial applications, for example, in civil engineering (heating/cooling of buildings) or cold storage applications. Another application may be the moderation of temperature increases in concrete encasements of radionuclides during their decay. The phase-change behavior of a material is determined by its heat capacity and the peak it exhibits near a phase change. We analyze the behavior of such peaks for a selected PCM at heating rates varying between \(0.1\,^{\circ }\hbox {C}\cdot \hbox {min}^{-1}\) and \(1\,^{\circ }\hbox {C}\cdot \hbox {min}^{-1}\), corresponding in real situations to different decay rates of radionuclides. We show that experimentally measured peaks can be plausibly described by an equilibrium theory that enables us to calculate the latent heat and phase-change temperature from experimental data.





Similar content being viewed by others
References
E. Oró, A. de Gracia, A. Castell, M.M. Farid, L.F. Cabeza, Appl. Energy 99, 513–533 (2012)
M. Kenisarin, K. Mahkamov, Renew. Sust. Energy Rev. 11, 1913–1965 (2007)
L.F. Cabeza, A. Castell, C. Barreneche, A. de Gracia, A.I. Fernández, Renew. Sust. Energy Rev. 15, 1675–1695 (2011)
F. Kuznik, D. David, K. Johannes, J.-J. Roux, Renew. Sust. Energy Rev. 15, 379–391 (2011)
N. Soares, J.J. Costa, A.R. Gaspar, P. Santos, Energy Build. 59, 82–103 (2013)
B. Pomaro, Model. Simul. Eng. 2016, 4165746 (2016)
I. Medved’, A. Trník, L. Vozár, Int. J. Heat Mass Transf. 107, 123–132 (2017)
E. Günther, S. Hiebler, H. Mehling, R. Redlich, Int. J. Thermophys. 30, 1257–1269 (2009)
C. Schick, Anal. Bioanal. Chem. 395, 1589–1611 (2009)
C.S.P. Tripathi, P. Losada-Pérez, C. Glorieux, A. Kohlmeier, M.-G. Tamba, G.H. Mehl, J. Leys, Phys. Rev. E 84, 041707 (2011)
O. Zmeškal, P. Štefková, L. Dohnalová, R. Bařinka, Int. J. Thermophys. 34, 926–938 (2013)
A. Eddhahak-Ouni, S. Drissi, J. Colin, J. Neji, S. Care, Appl. Therm. Eng. 64, 32–29 (2014)
J. Giro-Paloma, C. Barreneche, M. Martínez, B. Šumiga, L.F. Cabeza, A.I. Fernández, Energy 87, 223–227 (2015)
Standard DIN 51007:1994-06, General principles of differential thermal analysis (Beuth, Germany, 1994)
E. Gmelin, S.M. Sarge, Thermochim. Acta 347, 9–13 (2000)
Standard ISO 11357-1:2016, Plastics – differential scanning calorimetry (DSC)—part 1: General principles (ISO Committee, Geneve, 2016)
D. Lencer, M. Salinga, M. Wuttig, Adv. Mater. 23, 2030–2058 (2011)
Acknowledgements
This research was supported by the Czech Science Foundation, Project No. 17-11635S.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Medved’, I., Trník, A. Behavior of a PCM at Varying Heating Rates: Experimental and Theoretical Study with an Aim at Temperature Moderation in Radionuclide Concrete Encasements. Int J Thermophys 39, 83 (2018). https://doi.org/10.1007/s10765-018-2408-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-018-2408-x