Abstract
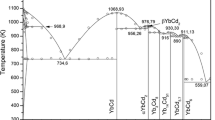
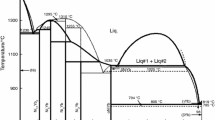
A calculation model of the Gibbs energy of ternary oxide compounds from the binary components was used. Thermodynamic properties of \(\mathrm{Yb}_{2} \mathrm{O}_{3}\)–\(\mathrm{Bi}_{2}\mathrm{O}_{3}\)–\(\mathrm{B}_{2}\mathrm{O}_{3}\) ternary systems in the condensed state were calculated. Thermodynamic data of binary and ternary compounds were used to determine the stable sections. The probability of reactions between the corresponding components in the \(\mathrm{Yb}_{2} \mathrm{O}_{3}\)–\(\mathrm{Bi}_{2} \mathrm{O}_{3}\)–\(\mathrm{B}_{2} \mathrm{O}_{3}\) system was estimated. Fusibility diagrams of systems \(\mathrm{BiBO}_{3}\)–\(\mathrm{YbBO}_{3}\) and \(\mathrm{Bi}_{4} \mathrm{B}_{2} \mathrm{O}_{9}\)–\(\mathrm{YbBO}_{3}\) were studied by physical–chemical analysis. The isothermal section of the phase diagram of \(\mathrm{Yb}_{2} \mathrm{O}_{3}\)–\(\mathrm{Bi}_{2} \mathrm{O}_{3}\)–\(\mathrm{B}_{2} \mathrm{O}_{3}\) at 298 K is built, as well as the projection of the liquid surface of \(\mathrm{BiBO}_{3}\)–\(\mathrm{B}_{2} \mathrm{O}_{3}\)–\(\mathrm{YbBO}_{3}\).




Similar content being viewed by others
References
A.V. Egorysheva, Yu.F. Kargin, Neorg. Mater. 34, 859 (1998) [in Russian]
A.V. Egorysheva, V.M. Skorikov, V.D. Volodin, O.E. Mislitskiy, Yu.F. Kargin, Zh. Neorg. Khim 51, 2078 (2006) [in Russian]
A.V. Egorysheva, V.D. Volodin, V.M. Skorikov, Neorg. Mater. 44, 76 (2008) [in Russian]
Yu.F. Kargin, S.N. Ivicheva, M.G. Komova, V.A. Krutko, Zh. Neorg. Khim 53, 478 (2008) [in Russian]
Yu.F. Kargin, S.N. Ivicheva, L.I. Shvorneva, Zh. Neorg. Khim 53, 1391 (2008) [in Russian]
A.V. Egorysheva, V.D. Volodin, V.M. Skorikov, Neorg. Mater. 44, 1397 (2008) [in Russian]
Yu.F. Kargin, S.N. Ivicheva, L.I. Shvorneva, M.G. Komova, V.A. Krutko, Zh. Neorg. Khim. 53, 1614 (2008) [in Russian]
A.V. Egorysheva, V.D. Volodin, V.M. Skorikov, G.Y. Yurkov, Neorg. Mater. 46, 495 (2010) [in Russian]
E.M. Levin, C.L. McDaniel, J. Am. Ceram. Soc. 45, 355 (1962)
M.I. Zargarova, N.A. Akhmedova, E.S. Guluzade, Zh. Neorg. Khim. 40, 1389 (1995)
The Landolt-Bornstein Database. Numerical Data and Functional Relationships in Science and Technology. Group III. Crystal Structure Data of Inorganic Compounds, vol. 7 (Springer, Berlin, 1987), p. 121
M. Drache, P. Roussel, J.P. Wignacourt, P. Conflant, Mater. Res. Bull. 39, 1393 (2004)
G. Yao, X. Wang, Y. Yang, L. Li, J. Am. Ceram. Soc. 93, 1697 (2010)
L.N. Dmitruk, O.B. Petrova, A.V. Popov, V.E. Shukshin, Trudy IOFRAN im. A.M.Prohorov 64, 49 (2008) [in Russian]
Termicheskie konstanty veschestv. Spravochnik / Pod red. V.P. Glushko (Moskva, VINITI AN SSSR 8, 535, 1978) [in Russian]; Thermal Constants of Substances. Handbook, ed. by V.P. Glushko (VINITI AS USSR, Moscow, 1978)
O. Kubaschewski, C.B. Alcook, Metallurgical Thermochemistry (Pergamon Press, Oxford, 1979)
V.T. Maltsev, S.A. Kutolin, Zh. Neorg. Khim. 24, 12 (1979) [in Russian]
P. Becker, Cryst. Res. Technol. 38, 74 (2003)
R. Ihara, T. Honma, Y. Benino, T. Fujiwara, T. Komatsu, Opt. Mater. 27, 403 (2004)
A.V. Egorysheva, V.I. Burkov, Yu.F. Kargin, V.G. Plotnichenko, V.V. Koltashev, Kristallografiya 50, 135 (2005) [in Russian]
A. Bajaj, A. Khanna, J. Phys. Condens. Matter. 21, 035112 (2009)
P. Becker, R. Frohlich, Z. Naturforsch. B 59, 256 (2004)
Yu.F. Kargin, V.P. Zhereb, A.V. Egorysheva, Zh. Neorg. Khim. 47, 992 (2002) [in Russian]
H. Huppertz, J. Chem. Sci. 56b, 697 (2001)
Diagrammi sostoyaniya system tugoplavkih oksidov. Spravochnik, Vip. 5, Dvoynie sistemi (Inst. Khimii silikatov, Nauka, Leningrad, 1985), p. 284 s [in Russian]; The Phase Diagrams of Systems of Refractory Oxides Handbook, vol. 5 (Institute of Silicate Chemistry, Science, Leningrad, 1985)
W.F. Bradley, D.L. Graf, R.S. Roth, Acta Cryst. 20, 283 (1966)
E.M. Levin, Phase Diagrams, vol. 3 (Academic Press, New York, 1970), p. 180
E.M. Levin, R.S. Roth, J.B. Martin, Am. Miner. 46, 1029 (1961)
M. Muehlberg, M. Burianek, H. Edongue, C. Poetsch, J. Cryst. Growth 237, 740 (2002)
M.M. Asadov, N.A. Akhmedova, Azerb. Khim. Zhurn. 3, 118 (2002) [in Azerbaijani]
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Asadov, M.M., Akhmedova, N.A. Fusion Diagrams in the \(\mathrm{BiBO}_{3}\)–\(\mathrm{YbBO}_{3}\) and \(\mathrm{Bi}_{4}\mathrm{B}_{2}\mathrm{O}_{9}\)–\(\mathrm{YbBO}_{3}\) Systems. Int J Thermophys 35, 1749–1756 (2014). https://doi.org/10.1007/s10765-014-1673-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10765-014-1673-6