Abstract
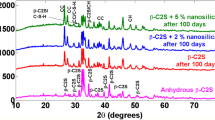
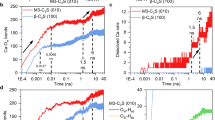
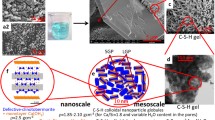
Cement hydration is a process involving simultaneous reactions of cement constituents, primarily tricalcium silicate (C3S) and dicalcium silicate (C2S), with the formation of key hydration products, calcium silicate hydrate (C–S–H) and calcium hydroxide (Ca(OH)2). Compared to the conventionally explored mid-infrared spectroscopy which is bond specific, terahertz (THz) spectroscopy is highly sensitive to crystalline arrangements and resonances in THz frequency range are primarily due to bulk vibrational modes. Hence, THz spectroscopy can be an effective complimentary tool to study the hydration process as C3S gets converted to different polymorphs of C–S–H. To understand the origin and variation of THz resonances of C3S, C–S–H polymorphs and Ca(OH)2, vibrational modes of C3S, tobemorite 9, tobermorite 14, jennite, and portlandite have been calculated using density functional theory simulations. The origin of the main resonances has been studied using vibrational density of states. Simulations show, for C3S, the resonance around 520 cm−1 appears due to combined effect of symmetric and asymmetric vibrations in SiO4 tetrahedra, the resonance around 450 cm−1 appears due to the combined effect of symmetric and asymmetric SiO4 tetrahedra, and CaO vibrations and the resonance around 318 cm−1 is primarily due to CaO vibrations. THz spectroscopy has been performed to track and understand the contribution of C3S in cement hydration. By combining the simulation and experiments, this work clearly explains the reduction of 520 cm−1 resonance, the constant intensity of 450 cm−1 resonance and frequency shift of the main resonances as C3S is transformed into various polymorphs of C–S–H during hydration.







Similar content being viewed by others
References
H.F.W. Taylor, “Cement Chemistry”. Thomas Telford Publishing, Thomas Telford Services Ltd., (1997).
F. Puertas, S. Goni, M.S. Hernandez, A. Geurrero, C. Varga, “Hydration of C3S, C2S and their Blends. Micro- and Nanoscale Characterization”. 13th International Congress on the Chemistry of Cement, 282 (2011).
W. Kurdowski, “Cement and Concrete Chemistry”. Springer (2014).
K. L. Scrivener, “Development of the microstructure during the hydration of Portland cement”. PhD Thesis, University of London (1984).
K. L. Scrivener, in: J.P. Skalny (Ed.), “The Microstucture of Concrete in Materials Science of Concrte I”. American Ceramic Society, Columbus, 127–161 (1989).
I. G. Richardson, “Electron Microscopy of cements”. in J. Bensted, P. Barnes (Eds.), “Structure and Performance of Cements”. Spon Press, London (2002).
K. L. Scrivener, T. Füllmann, E. Gallucci, G. Walenta, E. Bermejo, “Quantitaive study of Portland Cement hydration by X-ray Diffraction/Rietveld analysis and independent methods”. Cement and Concrete Research, 34, 1541–1547 (2004).
J. Elena, M. Daniela Lucia, “Application of X-Ray Diffraction (XRD) and Scanning Electron Microscopy (SEM) Methods to the Portland Cement Hydration Process”. Journal of Applied Engineering Sciences, 2(15), 35–42 (2012).
F. Kontoleontos, P. Tsakiridis, A. Marinos, N. Katsiotis, V. Kaloidas, M. Katsioti, “Dry-grinded Ultrafine Cements Hydration. Physicochemical and Microstructural Characterization”. Materials Research, 16(2), 404–416 (2013).
R. Ylmén, U. Jäglid, B-M. Steenari, I. Panas, “Early Hydration and setting of Portland cement monitored by IR, SEM and Vicat techniques”. Cement and Concrete Research, 39, 433–439 (2009).
R. Ylmén, U. Jäglid, I. Panas, “Monitoring Early Hydration of Cement by Ex Situ and In Situ ATR-FTIR – a Comparitive Study ”. Journal of the American Ceramic Society, 1–7 (2014).
R. Ylmén, U. Jäglid, “Carbonation of Portland Cement Studied by Diffuse Reflection Fourier Transform Infrared Spectroscopy”. International Journal of Concrete Structures and Materials, 7–2, 119–125 (2013).
R. Ylmén, “Early Hydration of Portland Cement – An Infrared Spectroscopy Perspective Complemented by Calorimetry and Scanning Electron Microscopy”. Doctoral Thesis, Chalmers University of Technology (2013).
R. Ylmén, L. Wadsö, I. Panas, “Insights into early hydration of Portland limestone cement from infrared spectroscopy and isothermal calorimetry”. Cement and Concrete Research, 40, 1541–1546 (2010).
A. Delgado, R. Paroli, J. Beaudoin, “Comparison of IR Techniques for the Characterization of Construction Cement Minerals and Hydrated Products”. Applied Spectroscopy, 50–8, 970–976 (1996).
M. Horgnies, J.J. Chen, C. Bouillon, “Cementitous materials overview about the use of Fourier Transform Infrared Spectroscopy to study cementitous materials”. Materials Characterisation VI: Computational Methods and Experiments, 77–5, Transactions on Engineering Sciences, WIT Press (2013).
I. Garcia-Lodeiro, A. Fernandez-Jimenez, A. Palomo, “Hydration Kinetics Hybrid Binders: Early Reaction Stages”. Cement and Concrete Composites, 39, 82–92 (2013).
S. Ray, J. Dash, N. Devi, S. Sasmal, B. Pesala, “Hydration Kinetics of Cement Composites with varying Water-Cement Ratio using Terahertz Spectroscopy”. Proc. SPIE 9362, Terahertz, RF, Millimeter, and Submillimeter-Wave Technology and Applications VIII, 936211 (2015).
M. Courtial, M. –N. de Noirfontaine, F. Dunstetter, G. Gasecki, M. Signes-Frehel, “Polymorphism of Tricalcium Silicate in Portland Cement: A Fast Visual Detection of Structure and Superstructure”. Powder Diffraction. 18–1, 7–15 (2003).
S.J. Clark, M.D. Segall, C.J. Pickard, P.J. Hasnip, M.J. Probert, K. Refson, M.C. Payne. Zeitschrift für Kristallographie, 220 (5–6), 567–570 (2005).
W.G. Mumme. “Crystal Structure of tricalcium silicate from a Portland cement clinker and its application to quantitaitve XRD analysis”.Neues Jahrbuch für Mineralogie. 4, 145–160 (2005).
E. Durgun, H. Manzano, R. J. M. Pellenq, J. C. Grossman, “Understanding and Controlling the Reactivity of the Calcium Silicate phases from First Principles”, Chemistry of Materials, 24 (7), 1262–1267 (2012).
I.F. Sáez del Bosque, S. Martínez-Ramírez, M.T. Blanco-Varela, “FTIR study of the effect of temperature and nanosilica on the nanostructure of C-S-H gel formed by hydrating tricalcium silicate”. Construction and Building Materials, 52, 314–323 (2014).
C. Biagioni, S. Merlino, E. Bonaccorsi, “The Tobermorite Subgroup: A New Nomenclature”. Minerological Magazine, 79(2), 485–495 (2015).
S. Merlino, E. Bonaccorsi, T. Armbruster, “Tobermorites: Their Real Structure and order-disorder (OD) character”. American Mineralogist, 84, 1613–1621 (1999).
E. Bonaccorsi, S. Merlino, A. R. Kampf, “The Crystal Structure of Tobermorite 14 Å (Plombierite), A C-S-H Phase”. Journal of the American Ceramic Society, 88–3, 505–512 (2005).
E. Bonaccorsi, S. Merlino, H.F.W. Taylor, “The Crystal Structure of Jennite, Ca9Si6O18(OH)6.8H2O”. Cement and Concrete Research, 34, 1481–1488 (2004).
D.M. Henderson, H.S. Gutiwsky, “A Nuclear Magnetic Resonance Determination of the Hydrogen Positions in Ca(OH)2”. The American Mineralogist, 47, 1231–1251 (1962).
J. P. Perdew, K. Burke, M. Ernzerhof, “Generalized gradient approximation made simple”. Physics Review Letters, 77, 3865–3868 (1996)
T, H, Fischer, J. Almlöf, “General Methods for gemoetry and wave function optimization”. Journal of Physical Chemistry, 96, 9768–9774, (1992).
P. Yu, R. J. Kirkpatrick, B. Poe, P.F. McMillan, X. Cong, “Structure of Calcium Silicate Hydrate (C-S-H): Near-, Mid-, and Far-Infrared Spectroscopy ”. Journal of the American Ceramic Society, 82–3, 742–748 (1999).
B. Gasharova, "Raman, conventional infrared and synchrotron infrared spectroscopy in mineralogy and geochemistry: basics and applications." Instrumental Techniques Applied to Mineralogy and Geochemistry”. I. Subias & B. Bauluz, eds. Seminarios SEM, 5, 5781 (2008).
A. Vidmer, G. Sclauzero, A. Pasquarello, “Infrared Spectra of jennite and tobermorite from first-principles”. Cement and Concrete Research, 60, 11–23 (2014).
T. N. Brusentsova, R. E. Peale, D. Maukonen, G. E. Harlow, J. S. Boesenberg, D. Ebel, “Far Infrared Spectroscopy of Carbonate Minerals”. American Mineralogist, 95, 1515–1522 (2010).
M. Handke, “Vibrational Spectra, Force Constants, and Si-O Bond Character in Calcium Silicate Crystal Structure”. Applied Spectroscopy, 40–6, 871–877 (1986).
K. C. Oppenheim, T. M. Korter, J. S. Melinger,‡ and D. Grischkowsky, “Solid-State Density Functional Theory Investigation of the Terahertz Spectra of the Structural Isomers 1,2-Dicyanobenzene and 1,3-Dicyanobenzene”. Journal of Physical Chemistry A, 114 (47), 12513–12521 (2010).
L. Ding, W-H. Fan, X. Chen, Z-Y Chen, C. Song, “Terahertz spectroscopy and solid-state density functional theory calculations of structural isomers: Nicotinic acid, isonicotinic acid and 2-picolinic acid”. Modern Physics Letters B, 31–13, (2017).
M. J. A. Qomi, F. -J. Ulm, R. J. -M. Pellenq, “Physical Origins of Thermal Properties of Cement Paste”. Physical Review Applied, 3, 064010 (2015).
M.E. Simonsen, C. Sønderby, Z. Li, E.G. Søgaard, “XPS and FTIR Investigation of Silicate Polymers”. Journal of Material Science, 44, 2079–2088 (2014).
L.P. Singh, W. Zhu, T. Howind, U. Sharma, “Quantification and characterization of C-S-H in silica nanoparticles incorporated in cementitious system”. Cement and Concrete Composites, 79, 106–116 (2017).
Acknowledgements
The authors would like to thank Director, CSIR-CEERI, Director, CSIR-SERC, Scientist-in-charge, CEERI, Chennai, for their support throughout the research work and acknowledge the financial support through CSIR network project CSC-0128 (FUTURE). The research work has been carried out with the equipment of CSIR Innovation Complex for which authors would like to thank Director, CSIR-SERC. Shaumik Ray would like to thank CSIR-Senior Research Fellowship for the financial support. The authors would like to thank Rikard Ylmen for his kind suggestions in sample preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ray, S., Dash, J., Devi, N. et al. Comparative Study of Hydration Kinetics of Cement and Tricalcium Silicate Using Terahertz Spectroscopy and Density Functional Theory Simulations. J Infrared Milli Terahz Waves 39, 651–666 (2018). https://doi.org/10.1007/s10762-018-0501-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10762-018-0501-7