Abstract
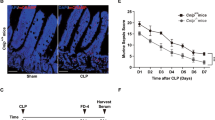
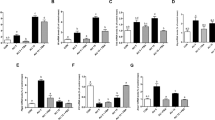
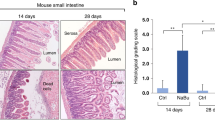
Sepsis is a life-threatening condition with a high rate of mortality. Unfortunately, very few therapies can improve outcomes in patients with sepsis. Butyrate, which is the most potent histone deacetylase (HDAC) inhibitor among short-chain fatty acids, exerts anti-inflammatory effects in a variety of inflammatory diseases. Butyrate might thus be valuable in the treatment of sepsis, in which inhibition of overwhelming cytokine release is vitally important. Sepsis was induced in 7- to 8-week-old Sprague-Dawley rats by cecal ligation and puncture (CLP) with a 21-g double-puncture technique. Rats received an intravenous injection of normal saline (vehicle) or sodium butyrate (200 mg/kg) after CLP and were sacrificed 12 h later. Hematoxylin and eosin staining was performed to observe the intestinal mucosal morphology. RT-PCR and ELISA were used to determine the intestinal inflammatory response in vivo. Intestinal permeability was evaluated by measuring fluorescein isothiocyanate dextran (FD-4) absorption in vivo, and tight junction protein expression was examined by western blot. NF-κB p65 activities were assessed by western blot and immunohistochemistry. Sodium butyrate treatment improved the survival rate of CLP rats and alleviated sepsis-induced intestinal mucosal injury. Proinflammatory cytokine expression was lower in butyrate-treated rats than in the vehicle group. FD-4 leakage from the intestinal tract was reduced, and the expression levels of the tight junction proteins claudin-1 and ZO-1 were also restored in rats that received sodium butyrate treatment. These effects were associated with less NF-κB p65 nuclear translocation, whereas the expression of Iκ-Bα was not affected or even increased. Sodium butyrate mitigates the inflammatory response and maintains intestinal barrier function in polymicrobial sepsis partly through inhibition of NF-κB activation and may serve as a novel therapy for sepsis.






Similar content being viewed by others
Abbreviations
- CLP:
-
Cecal ligation and puncture
- NaB:
-
Sodium butyrate
- NF-κB:
-
Nuclear factor-kappa B
References
Dellinger, R.P., M.M. Levy, A. Rhodes, et al. 2013. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Medicine 39: 165–228.
Hotchkiss, R.S., G. Monneret, and D. Payen. 2013. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nature Reviews. Immunology 13: 862–874.
Assimakopoulos, S.F., C. Triantos, K. Thomopoulos, F. Fligou, I. Maroulis, M. Marangos, and C.A. Gogos. 2018. Gut-origin sepsis in the critically ill patient: Pathophysiology and treatment. Infection. 46: 751–760.
Klingensmith, N.J., and C.M. Coopersmith. 2016. The gut as the motor of multiple organ dysfunction in critical illness. Critical Care Clinics 32: 203–212.
Scheppach, W. 1994. Effects of short chain fatty acids on gut morphology and function. Gut. 35: S35–S38.
Vinolo, M.A., H.G. Rodrigues, R.T. Nachbar, et al. 2011. Regulation of inflammation by short chain fatty acids. Nutrients. 3: 858–876.
Vieira, E.L., A.J. Leonel, A.P. Sad, et al. 2012. Oral administration of sodium butyrate attenuates inflammation and mucosal lesion in experimental acute ulcerative colitis. The Journal of Nutritional Biochemistry 23: 430–436.
Sun, J., F. Wang, H. Li, et al. 2015. Neuroprotective effect of sodium butyrate against cerebral ischemia/reperfusion injury in mice. BioMed Research International 2015: 395895.
Qiao, Y.L., J.M. Qian, F.R. Wang, Z.Y. Ma, and Q.W. Wang. 2014. Butyrate protects liver against ischemia reperfusion injury by inhibiting nuclear factor kappa B activation in Kupffer cells. The Journal of Surgical Research 187: 653–659.
Correa-Oliveira, R., J.L. Fachi, A. Vieira, et al. 2016. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunology. 5: e73.
Liu, J., F. Wang, H. Luo, A. Liu, K. Li, C. Li, and Y. Jiang. 2016. Protective effect of butyrate against ethanol-induced gastric ulcers in mice by promoting the anti-inflammatory, anti-oxidant and mucosal defense mechanisms. International Immunopharmacology 30: 179–187.
Zhang, L.T., Y.M. Yao, J.Q. Lu, X.J. Yan, Y. Yu, and Z.Y. Sheng. 2007. Sodium butyrate prevents lethality of severe sepsis in rats. Shock. 27: 672–677.
Zhang, L., S. Jin, C. Wang, R. Jiang, and J. Wan. 2010. Histone deacetylase inhibitors attenuate acute lung injury during cecal ligation and puncture-induced polymicrobial sepsis. World Journal of Surgery 34: 1676–1683.
Mishra, S.K., and S. Choudhury. 2018. Experimental protocol for cecal ligation and puncture model of polymicrobial sepsis and assessment of vascular functions in mice. Methods in Molecular Biology 1717: 161–187.
Chiu, C.J., A.H. McArdle, R. Brown, et al. 1970. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Archives of Surgery 101: 478–483.
Kao, N.R., A. Xenocostas, D.K. Driman, et al. 2011. Recombinant human erythropoietin improves gut barrier function in a hemorrhagic shock and resuscitation rat model. The Journal of Trauma 71: S456–S461.
Meng, M., N.J. Klingensmith, and C.M. Coopersmith. 2017. New insights into the gut as the driver of critical illness and organ failure. Current Opinion in Critical Care 23: 143–148.
Ni, Y.F., J. Wang, X.L. Yan, et al. 2010. Histone deacetylase inhibitor, butyrate, attenuates lipopolysaccharide-induced acute lung injury in mice. Respiratory Research 11.
Wang, F., Z. Jin, K. Shen, T. Weng, Z. Chen, J. Feng, Z. Zhang, J. Liu, X. Zhang, and M. Chu. 2017. Butyrate pretreatment attenuates heart depression in a mice model of endotoxin-induced sepsis via anti-inflammation and anti-oxidation. The American Journal of Emergency Medicine 35: 402–409.
Dejager, L., I. Pinheiro, E. Dejonckheere, and C. Libert. 2011. Cecal ligation and puncture: The gold standard model for polymicrobial sepsis? Trends in Microbiology 19: 198–208.
Gentile, L.F., A.G. Cuenca, E.L. Vanzant, P.A. Efron, B. McKinley, F. Moore, and L.L. Moldawer. 2013. Is there value in plasma cytokine measurements in patients with severe trauma and sepsis? Methods. 61: 3–9.
Aksoy, A.N., A. Toker, M. Celik, et al. 2014. The effect of progesterone on systemic inflammation and oxidative stress in the rat model of sepsis. Indian Journal of Pharmacology 46: 622–626.
Huang, B., X.D. Yang, A. Lamb, and L.F. Chen. 2010. Posttranslational modifications of NF-kappaB: Another layer of regulation for NF-kappaB signaling pathway. Cellular Signalling 22: 1282–1290.
Liu, S.F., and A.B. Malik. 2006. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. American Journal of Physiology. Lung Cellular and Molecular Physiology 290: L622–L645.
Li, Q., Q. Zhang, C. Wang, X. Liu, N. Li, and J. Li. 2009. Disruption of tight junctions during polymicrobial sepsis in vivo. The Journal of Pathology 218: 210–221.
Lechuga, S., and A.I. Ivanov. 2017. Disruption of the epithelial barrier during intestinal inflammation: Quest for new molecules and mechanisms. Biochimica et Biophysica Acta, Molecular Cell Research 1864: 1183–1194.
Berkes, J., V.K. Viswanathan, S.D. Savkovic, and G. Hecht. 2003. Intestinal epithelial responses to enteric pathogens: Effects on the tight junction barrier, ion transport, and inflammation. Gut. 52: 439–451.
Singh, N., M. Thangaraju, P.D. Prasad, P.M. Martin, N.A. Lambert, T. Boettger, S. Offermanns, and V. Ganapathy. 2010. Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. The Journal of Biological Chemistry 285: 27601–27608.
Park, J., M. Kim, S.G. Kang, A.H. Jannasch, B. Cooper, J. Patterson, and C.H. Kim. 2015. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunology 8: 80–93.
Ito, K. 2007. Impact of post-translational modifications of proteins on the inflammatory process. Biochemical Society Transactions 35: 281–283.
Flint, H.J., K.P. Scott, P. Louis, and S.H. Duncan. 2012. The role of the gut microbiota in nutrition and health. Nature Reviews. Gastroenterology & Hepatology 9: 577–589.
Kiernan, R., V. Bres, R.W. Ng, et al. 2003. Post-activation turn-off of NF-kappa B-dependent transcription is regulated by acetylation of p65. The Journal of Biological Chemistry 278: 2758–2766.
Ziesche, E., D. Kettner-Buhrow, A. Weber, et al. 2013. The coactivator role of histone deacetylase 3 in IL-1-signaling involves deacetylation of p65 NF-kappaB. Nucleic Acids Research 41: 90–109.
Leus, N.G., M.R. Zwinderman, and F.J. Dekker. 2016. Histone deacetylase 3 (HDAC 3) as emerging drug target in NF-kappaB-mediated inflammation. Current Opinion in Chemical Biology 33: 160–168.
Li, H., W. Han, V. Polosukhin, et al. 2013. NF-kappaB inhibition after cecal ligation and puncture reduces sepsis-associated lung injury without altering bacterial host defense. Mediators of Inflammation 503213: 2013.
Chen, L.F., and W.C. Greene. 2004. Shaping the nuclear action of NF-kappaB. Nature Reviews. Molecular Cell Biology 5: 392–401.
Kaplan, J., M. Nowell, R. Chima, and B. Zingarelli. 2014. Pioglitazone reduces inflammation through inhibition of NF-kappaB in polymicrobial sepsis. Innate Immunity 20: 519–528.
Ghizzoni, M., H.J. Haisma, H. Maarsingh, and F.J. Dekker. 2011. Histone acetyltransferases are crucial regulators in NF-kappaB mediated inflammation. Drug Discovery Today 16: 504–511.
Spange, S., T. Wagner, T. Heinzel, and O.H. Krämer. 2009. Acetylation of non-histone proteins modulates cellular signalling at multiple levels. The International Journal of Biochemistry & Cell Biology 41: 185–198.
Choo, Q.Y., P.C. Ho, and H.S. Lin. 2008. Histone deacetylase inhibitors: New hope for rheumatoid arthritis? Current Pharmaceutical Design 14: 803–820.
Egorin, M.J., Z.M. Yuan, D.L. Sentz, K. Plaisance, and J.L. Eiseman. 1999. Plasma pharmacokinetics of butyrate after intravenous administration of sodium butyrate or oral administration of tributyrin or sodium butyrate to mice and rats. Cancer Chemotherapy and Pharmacology 43: 445–453.
Qiao, Y., J. Qian, Q. Lu, Y. Tian, Q. Chen, and Y. Zhang. 2015. Protective effects of butyrate on intestinal ischemia-reperfusion injury in rats. The Journal of Surgical Research 197: 324–330.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fu, J., Li, G., Wu, X. et al. Sodium Butyrate Ameliorates Intestinal Injury and Improves Survival in a Rat Model of Cecal Ligation and Puncture-Induced Sepsis. Inflammation 42, 1276–1286 (2019). https://doi.org/10.1007/s10753-019-00987-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-00987-2