Abstract
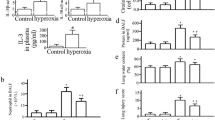
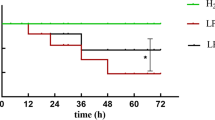
Hyperoxic acute lung injury is a serious complication of oxygen therapy that causes high mortality. Inhibition of soluble epoxide hydrolase (sEH) has been reported to have protective effect on lipopolysaccharide-induced acute lung injury (ALI). This study investigates whether sEH plays any role in the pathogenesis of hyperoxic ALI. Wild-type and sEH gene knockout (sEH−/−) mice were exposed to 100% O2 for 72 h to induce hyperoxic ALI. Hyperoxia caused infiltration of inflammatory cells, elevation of interleukin-1β and interleukin-6 levels, and deterioration of alveolar capillary protein leak as well as wet/dry weight ratio in the lung. The hyperoxia-induced pulmonary inflammation and edema were markedly improved in sEH−/− mice. Survival rate was significantly improved in sEH−/− mice compared with that in wild-type mice. Moreover, the levels of epoxyeicosatrienoic acids and heme oxygenase-1 activity were notably elevated in sEH−/− mice compared with those in wild-type mice after exposure to 100% O2 for 72 h. The nucleotide-binding domains and leucine-rich repeat pyrin domains containing 3 (NLRP3) inflammasome activation and caspase-1 activity induced by hyperoxia were inhibited in sEH−/− mice compared with those in wild-type mice. Inhibition of sEH by an inhibitor, AUDA, dampened hyperoxia-induced ALI. sEH plays a vital role in hyperoxic ALI and is a potential therapeutic target for ALI.



Similar content being viewed by others
References
Cochrane, C.G., R. Spragg, and S.D. Revak. 1983. Pathogenesis of the adult respiratory distress syndrome. Evidence of oxidant activity in bronchoalveolar lavage fluid. The Journal of Clinical Investigation 71: 754–761.
Leaver, S.K., and T.W. Evans. 2007. Acute respiratory distress syndrome. BMJ 335: 389–394.
Sweeney, R.M., and D.F. McAuley. 2016. Acute respiratory distress syndrome. Lancet 388: 2416–2430.
Tao, W., Q.B. Miao, Y.B. Zhu, and Y.S. Shu. 2012. Inhaled neutrophil elastase inhibitor reduces oleic acid-induced acute lung injury in rats. Pulmonary Pharmacology & Therapeutics 25: 99–103.
Ganter, M.T., J. Roux, B. Miyazawa, M. Howard, J.A. Frank, G. Su, et al. 2008. Interleukin-1beta causes acute lung injury via alphavbeta5 and alphavbeta6 integrin-dependent mechanisms. Circulation Research 102: 804–812.
Fukumoto, J., I. Fukumoto, P.T. Parthasarathy, R. Cox, B. Huynh, G.K. Ramanathan, et al. 2013. NLRP3 deletion protects from hyperoxia-induced acute lung injury. American Journal of Physiology. Cell Physiology 305: C182–C189.
Tao, W., P.S. Li, L.Q. Yang, and Y.B. Ma. 2016. Effects of a soluble epoxide hydrolase inhibitor on lipopolysaccharide-induced acute lung injury in mice. PLoS One 11: e0160359.
Chen, X., X. Zhang, J. Zhang, Y. Gao, Z. Yang, S. Li, et al. 2017. Attenuation of acute lung injury in a rat model by Semen Cassiae. BMC Complementary and Alternative Medicine 17: 234.
Smith, K.R., K.E. Pinkerton, T. Watanabe, T.L. Pedersen, S.J. Ma, and B.D. Hammock. 2005. Attenuation of tobacco smoke-induced lung inflammation by treatment with a soluble epoxide hydrolase inhibitor. Proceedings of the National Academy of Sciences of the United States of America 102: 2186–2191.
Zhou, Y., J. Yang, G.Y. Sun, T. Liu, J.X. Duan, H.F. Zhou, et al. 2016. Soluble epoxide hydrolase inhibitor 1-trifluoromethoxyphenyl-3- (1-propionylpiperidin-4-yl) urea attenuates bleomycin-induced pulmonary fibrosis in mice. Cell and Tissue Research 363: 399–409.
Larsson, C., I. White, C. Johansson, A. Stark, and J. Meijer. 1995. Localization of the human soluble epoxide hydrolase gene (EPHX2) to chromosomal region 8p21-p12. Human Genetics 95: 356–358.
Tanaka, H., S.G. Kamita, N.M. Wolf, T.R. Harris, Z. Wu, C. Morisseau, et al. 2008. Transcriptional regulation of the human soluble epoxide hydrolase gene EPHX2. Biochimica et Biophysica Acta 1779: 17–27.
Newman, J.W., C. Morisseau, and B.D. Hammock. 2005. Epoxide hydrolases: Their roles and interactions with lipid metabolism. Progress in Lipid Research 44: 1–51.
Imig, J.D., K.A. Walsh, M.A. Hye Khan, T. Nagasawa, M. Cherian-Shaw, S.M. Shaw, et al. 2012. Soluble epoxide hydrolase inhibition and peroxisome proliferator activated receptor gamma agonist improve vascular function and decrease renal injury in hypertensive obese rats. Experimental Biology and Medicine (Maywood, N.J.) 237 (12): 1402.
Li, Y., G. Yu, S. Yuan, C. Tan, P. Lian, L. Fu, et al. 2017. Cigarette smoke-induced pulmonary inflammation and autophagy are attenuated in Ephx2-deficient mice. Inflammation 40: 497–510.
Motoki, A., M.J. Merkel, W.H. Packwood, Z. Cao, L. Liu, J. Iliff, et al. 2008. Soluble epoxide hydrolase inhibition and gene deletion are protective against myocardial ischemia-reperfusion injury in vivo. American Journal of Physiology. Heart and Circulatory Physiology 295: H2128–H2134.
Zhu, Y., M. Blum, U. Hoff, T. Wesser, M. Fechner, C. Westphal, et al. 2016. Renal ischemia/reperfusion injury in soluble epoxide hydrolase-deficient mice. PLoS One 11: e0145645.
Bettaieb, A., C. Morisseau, B. Hammock, and F. Haj. 2014. Soluble epoxide hydrolase deficiency ameliorates acute pancreatitis in mice. Free Radic Biol Med 75 (Suppl 1): S32.
Sinal, C.J., M. Miyata, M. Tohkin, K. Nagata, J.R. Bend, and F.J. Gonzalez. 2000. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. The Journal of Biological Chemistry 275: 40504–40510.
Tao, W., Y.S. Shu, Q.B. Miao, and Y.B. Zhu. 2012. Attenuation of hyperoxia-induced lung injury in rats by adrenomedullin. Inflammation 35: 150–157.
Bachofen, M., M. Bachofen, E.R. Weibel, and E.R. Weibel. 1982. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clinics in Chest Medicine 3: 35–56.
Jin, S.W., L. Zhang, Q.Q. Lian, D. Liu, P. Wu, S.L. Yao, et al. 2007. Posttreatment with aspirin-triggered lipoxin A4 analog attenuates lipopolysaccharide-induced acute lung injury in mice: The role of heme oxygenase-1. Anesthesia and Analgesia 104: 369–377.
Schmelzer, K.R., L. Kubala, J.W. Newman, I.H. Kim, J.P. Eiserich, and B.D. Hammock. 2005. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proceedings of the National Academy of Sciences of the United States of America 102: 9772–9777.
Liu, Y., H.K. Webb, H. Fukushima, J. Micheli, S. Markova, J.L. Olson, and D.L. Kroetz. 2012. Attenuation of cisplatin-induced renal injury by inhibition of soluble epoxide hydrolase involves nuclear factor kappaB signaling. The Journal of Pharmacology and Experimental Therapeutics 341: 725–734.
Bettaieb, A., S. Chahed, G. Tabet, J. Yang, C. Morisseau, S. Griffey, et al. 2014. Effects of soluble epoxide hydrolase deficiency on acute pancreatitis in mice. PLoS One 9: e113019.
Node, K., Y. Huo, X. Ruan, B. Yang, M. Spiecker, K. Ley, et al. 1999. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 285: 1276–1279.
Fan, J., R.D. Ye, and A.B. Malik. 2001. Transcriptional mechanisms of acute lung injury. American Journal of Physiology. Lung Cellular and Molecular Physiology 281: L1037–L1050.
Saleh, M. 2011. The machinery of Nod-like receptors: Refining the paths to immunity and cell death. Immunological Reviews 243: 235–246.
Martinon, F., K. Burns, and J. Tschopp. 2002. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Molecular Cell 10: 417–426.
Ogura, Y., F.S. Sutterwala, and R.A. Flavell. 2006. The inflammasome: First line of the immune response to cell stress. Cell 126: 659–662.
Galam, L., A. Rajan, A. Failla, R. Soundararajan, R.F. Lockey, and N. Kolliputi. 2016. Deletion of P2X7 attenuates hyperoxia-induced acute lung injury via inflammasome suppression. American Journal of Physiology. Lung Cellular and Molecular Physiology 310: L572–L581.
Dostert, C., V. Petrilli, R. Van Bruggen, C. Steele, B.T. Mossman, and J. Tschopp. 2008. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320: 674–677.
Liu, Y., X. Lu, S. Nguyen, J.L. Olson, H.K. Webb, and D.L. Kroetz. 2013. Epoxyeicosatrienoic acids prevent cisplatin-induced renal apoptosis through a p38 mitogen-activated protein kinase-regulated mitochondrial pathway. Molecular Pharmacology 84: 925–934.
Zhang, Y., G. Jiang, M. Sauler, and P.J. Lee. 2013. Lung endothelial HO-1 targeting in vivo using lentiviral miRNA regulates apoptosis and autophagy during oxidant injury. The FASEB Journal 27: 4041–4058.
Yu, J., Y. Wang, Z. Li, S. Dong, D. Wang, L. Gong, et al. 2016. Effect of heme oxygenase-1 on mitofusin-1 protein in LPS-induced ALI/ARDS in rats. Scientific Reports 6: 36530.
Vanella, L., D.H. Kim, K. Sodhi, I. Barbagallo, A.P. Burgess, J.R. Falck, et al. 2011. Crosstalk between EET and HO-1 downregulates Bach1 and adipogenic marker expression in mesenchymal stem cell derived adipocytes. Prostaglandins & Other Lipid Mediators 96: 54–62.
Elmarakby, A.A., J. Faulkner, C. Pye, K. Rouch, A. Alhashim, K.R. Maddipati, et al. 2013. Role of haem oxygenase in the renoprotective effects of soluble epoxide hydrolase inhibition in diabetic spontaneously hypertensive rats. Clinical Science (London, England) 125: 349–359.
Acknowledgements
We thank Sun Liu and Xuan Jiang for critical reading of the manuscript, and Qin Xiao and Yue-Xiang Chen for technical assistance.
Author information
Authors and Affiliations
Contributions
PSL, WT, and YSS conceived and designed the experiments.
WT and LQY performed the experiments.
WT and LQY analyzed data.
WT drafted the manuscript.
LQY edited and revised the manuscript.
PSL and WT interpreted results of the experiments.
PSL, WT, LQY, and YSS approved the final version of the manuscript.
Corresponding authors
Ethics declarations
All studies were performed in accordance with the National Institutes of Health guidelines for the use of experimental animals. The current project was approved by the Ethics Committee of Animal Research of Yangzhou University.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, PS., Tao, W., Yang, LQ. et al. Effect of Soluble Epoxide Hydrolase in Hyperoxic Acute Lung Injury in Mice. Inflammation 41, 1065–1072 (2018). https://doi.org/10.1007/s10753-018-0758-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0758-y