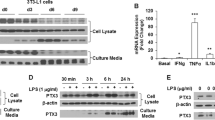
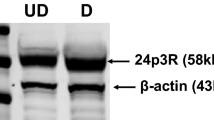
Abstract
Adipose tissue expansion is accompanied by infiltration and accumulation of pro-inflammatory macrophages, which links obesity to pathologic conditions such as type 2 diabetes. However, little is known regarding the role of pro-inflammatory adipose tissue remodeling in the thermogenic activation of brown/beige fat. Here, we investigated the effect of pattern recognition receptors (PRR) activation in macrophages, especially the toll-like receptor 4 (TLR4) and Nod-like receptor 3 (NLRP3), on white adipocyte browning. We report that TLR4 activation by lipopolysaccharide repressed white adipocyte browning in response to β3-adrenergic receptor activation and caused ROS production and mitochondrial dysfunction, while genetic deletion of TLR4 protected mitochondrial function and thermogenesis. In addition, activation of NLRP3 inflammasome in macrophages attenuated UCP1 induction and mitochondrial respiration in cultures of primary adipocytes, while the absence of NLRP3 protected UCP1 in adipocytes. The effect of NLRP3 inflammasome activation on browning was mediated by IL-1β signaling, as blocking IL-1 receptor in adipocytes protected thermogenesis. We also report that IL-1β interferes with thermogenesis via oxidative stress stimulation and mitochondrial dysfunction as we observed a statistically significant increase in ROS production, decrease in SOD enzyme activity, and increase in mitochondrial depolarization in adipocytes treated with IL-1β. Collectively, we demonstrated that inflammatory response to obesity, such as TLR4 and NLRP3 inflammasome activation as well as IL-1β secretion, attenuates β3-adrenoreceptor-induced beige adipocyte formation via oxidative stress and mitochondrial dysfunction. Our findings provide insights into targeting innate inflammatory system for enhancement of the adaptive thermogenesis against obesity.

Similar content being viewed by others
Abbreviations
- NLRP3:
-
NOD-like receptor protein 3
- TLR4:
-
Toll-like receptor 4
- WAT:
-
White adipose tissue
- PRR:
-
Pattern recognition receptors
- β3-AR:
-
Beta 3-adrenergic receptor
- ROS:
-
Reactive oxygen species
- UCP1:
-
Uncoupling protein 1
- IL-1β:
-
Interleukin-1 beta
- BMP7:
-
Bone morphogenetic protein 7
- LPS:
-
Lipopolysaccharide
- CL:
-
CL 316,243
- hASCs:
-
Human adipose-derived stem cells
- EMSC:
-
Ear mesenchymal stem cells
- sWAT:
-
Subcutaneous white adipose tissue
- mtDNA:
-
Mitochondrial DNA
- OCR:
-
Oxygen consumption rate
- SOD:
-
Superoxide dismutase
- hIL-1β-CM:
-
Human interleukin-1 beta-conditioned medium
- mIL-1β-CM:
-
Mouse interleukin-1 beta-conditioned medium
- NAC:
-
n-Acetylcysteine
- Bt2-cAMP:
-
Dibutyryl-cAMP
- IL-1RA:
-
IL-1 receptor antagonist
- MQ:
-
Macrophages
References
Alexaki, V.I., and T. Chavakis. 2016. The role of innate immunity in the regulation of brown and beige adipogenesis. Reviews in endocrine & metabolic disorders 17 (1): 41–49.
Reitman, M.L. 2017. How does fat transition from white to beige? Cell metabolism 26 (1): 14–16.
Qiu, Y., K.D. Nguyen, J.I. Odegaard, X. Cui, X. Tian, R.M. Locksley, et al. 2014. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 157 (6): 1292–1308.
Nguyen, K.D., Y. Qiu, X. Cui, Y.P. Goh, J. Mwangi, T. David, et al. 2011. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480 (7375): 104–108.
Chmelar, J., K.J. Chung, and T. Chavakis. 2013. The role of innate immune cells in obese adipose tissue inflammation and development of insulin resistance. Thrombosis and haemostasis 109 (3): 399–406.
Lackey, D.E., and J.M. Olefsky. 2016. Regulation of metabolism by the innate immune system. Nature reviews Endocrinology 12 (1): 15–28.
Thyagarajan, B., and M.T. Foster. 2017. Beiging of white adipose tissue as a therapeutic strategy for weight loss in humans. Hormone molecular biology and clinical investigation.
van Marken Lichtenbelt, W.D., J.W. Vanhommerig, N.M. Smulders, J.M. Drossaerts, G.J. Kemerink, N.D. Bouvy, et al. 2009. Cold-activated brown adipose tissue in healthy men. The New England Journal of Medicine 360 (15): 1500–1508.
Saito, M., Y. Okamatsu-Ogura, M. Matsushita, K. Watanabe, T. Yoneshiro, J. Nio-Kobayashi, et al. 2009. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58 (7): 1526–1531.
Vijgen, G.H., N.D. Bouvy, G.J. Teule, B. Brans, P. Schrauwen, and W.D. van Marken Lichtenbelt. 2011. Brown adipose tissue in morbidly obese subjects. PLoS One 6 (2): e17247.
Cypess, A.M., S. Lehman, G. Williams, I. Tal, D. Rodman, A.B. Goldfine, et al. 2009. Identification and importance of brown adipose tissue in adult humans. The New England journal of medicine 360 (15): 1509–1517.
Martins, F.F., T.C.L. Bargut, M.B. Aguila, and C.A. Mandarim-de-Lacerda. 2017. Thermogenesis, fatty acid synthesis with oxidation, and inflammation in the brown adipose tissue of ob/ob (−/−) mice. Annals of anatomy. Anatomischer Anzeiger : official organ of the Anatomische Gesellschaft 210: 44–51.
Okla, M., W. Wang, I. Kang, A. Pashaj, T. Carr, and S. Chung. 2015. Activation of Toll-like receptor 4 (TLR4) attenuates adaptive thermogenesis via endoplasmic reticulum stress. The Journal of Biological Chemistry 290 (44): 26476–26490.
Lumeng, C.N., J.L. Bodzin, and A.R. Saltiel. 2007. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of Clinical Investigation 117 (1): 175–184.
Sakamoto, T., N. Takahashi, Y. Sawaragi, S. Naknukool, R. Yu, T. Goto, et al. 2013. Inflammation induced by RAW macrophages suppresses UCP1 mRNA induction via ERK activation in 10T1/2 adipocytes. American Journal of Physiology Cell Physiology 304 (8): C729–C738.
Goto, T., S. Naknukool, R. Yoshitake, Y. Hanafusa, S. Tokiwa, Y. Li, et al. 2016. Proinflammatory cytokine interleukin-1beta suppresses cold-induced thermogenesis in adipocytes. Cytokine 77: 107–114.
Crane, J.D., E.P. Mottillo, T.H. Farncombe, K.M. Morrison, and G.R. Steinberg. 2014. A standardized infrared imaging technique that specifically detects UCP1-mediated thermogenesis in vivo. Molecular Metabolism 3 (4): 490–494.
Okla, M., J.H. Ha, R.E. Temel, and S. Chung. 2015. BMP7 drives human adipogenic stem cells into metabolically active beige adipocytes. Lipids 50 (2): 111–120.
Kim, J., M. Okla, A. Erickson, T. Carr, S.K. Natarajan, and S. Chung. 2016. Eicosapentaenoic acid potentiates brown thermogenesis through FFAR4-dependent up-regulation of miR-30b and miR-378. The Journal of Biological Chemistry 291 (39): 20551–20562.
Kim, Y., W. Wang, M. Okla, I. Kang, R. Moreau, and S. Chung. 2016. Suppression of NLRP3 inflammasome by gamma-tocotrienol ameliorates type 2 diabetes. Journal of Lipid Research 57 (1): 66–76.
Lumeng, C.N. 2013. Innate immune activation in obesity. Molecular Aspects of Medicine 34 (1): 12–29.
Hotamisligil, G.S. 2006. Inflammation and metabolic disorders. Nature 444 (7121): 860–867.
Pelicano, H., W. Lu, Y. Zhou, W. Zhang, Z. Chen, Y. Hu, et al. 2009. Mitochondrial dysfunction and reactive oxygen species imbalance promote breast cancer cell motility through a CXCL14-mediated mechanism. Cancer Research 69 (6): 2375–2383.
Birben, E., U.M. Sahiner, C. Sackesen, S. Erzurum, and O. Kalayci. 2012. Oxidative stress and antioxidant defense. The World Allergy Organization Journal 5 (1): 9–19.
Vandanmagsar, B., Y.H. Youm, A. Ravussin, J.E. Galgani, K. Stadler, R.L. Mynatt, et al. 2011. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nature Medicine 17 (2): 179–188.
Tack, C.J., R. Stienstra, L.A. Joosten, and M.G. Netea. 2012. Inflammation links excess fat to insulin resistance: the role of the interleukin-1 family. Immunological Reviews 249 (1): 239–252.
Wen, H., D. Gris, Y. Lei, S. Jha, L. Zhang, M.T. Huang, et al. 2011. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nature Immunology 12 (5): 408–415.
Cedikova, M., M. Kripnerova, J. Dvorakova, P. Pitule, M. Grundmanova, V. Babuska, et al. 2016. Mitochondria in white, brown, and beige adipocytes. Stem cells International 2016: 6067349.
Stienstra, R., C.J. Tack, T.D. Kanneganti, L.A. Joosten, and M.G. Netea. 2012. The inflammasome puts obesity in the danger zone. Cell Metabolism 15 (1): 10–18.
Benetti, E., F. Chiazza, N.S. Patel, and M. Collino. 2013. The NLRP3 Inflammasome as a novel player of the intercellular crosstalk in metabolic disorders. Mediators of Inflammation 2013: 678627.
Bae, J., J. Chen, and L. Zhao. 2015. Chronic activation of pattern recognition receptors suppresses brown adipogenesis of multipotent mesodermal stem cells and brown pre-adipocytes. Biochemistry and cell biology = Biochimie et biologie cellulaire 93 (3): 251–261.
Finlin, B.S., B. Zhu, A.L. Confides, P.M. Westgate, B.D. Harfmann, E.E. Dupont-Versteegden, et al. 2017. Mast cells promote seasonal white adipose beiging in humans. Diabetes 66 (5): 1237–1246.
Dowal, L., P. Parameswaran, S. Phat, S. Akella, I.D. Majumdar, J. Ranjan, et al. 2017. Intrinsic properties of brown and white adipocytes have differential effects on macrophage inflammatory responses. Mediators of Inflammation 2017: 9067049.
Bi, P., T. Shan, W. Liu, F. Yue, X. Yang, X.R. Liang, et al. 2014. Inhibition of Notch signaling promotes browning of white adipose tissue and ameliorates obesity. Nature Medicine 20 (8): 911–918.
Altshuler-Keylin S, Kajimura S. Mitochondrial homeostasis in adipose tissue remodeling. Science signaling. 2017;10(468).
Zhang, Y., S. Goldman, R. Baerga, Y. Zhao, M. Komatsu, and S. Jin. 2009. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proceedings of the National Academy of Sciences of the United States of America 106 (47): 19860–19865.
Acknowledgements
The authors wish to acknowledge the College of Medicine Research Center (CMRC) at King Saud University (KSU) and the Stem cell lab in the College of Medicine at KSU for providing access to their facilities to perform mitochondrial depolarization and oxidative stress experiments. Also, the authors wish to thank Rabih Halwani at the College of Medicine in KSU for permitting the use of Muse flow cytometry.
Funding
This study was supported by the National Institute of Health (Grant 1P20GM104320, project 5; to S. C.) in the USA, International Scientific Partnership Program (ISPP, no. 0103) at King Saud University (KSU) in Saudi Arabia, and Deanship of Scientific Research (Project No. R6-17-02-36) at King Saud University in Saudi Arabia.
Author information
Authors and Affiliations
Contributions
M.O. designed and performed experiments, analyzed data, and wrote the manuscript. W.Z. and M.A. critically reviewed the manuscript. S.C. provided scientific guidance, participated in discussions and manuscript preparation, and helped in interpreting the significance of the results.
Corresponding author
Ethics declarations
All protocols and procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Nebraska-Lincoln in Nebraska, United States, and by the Institutional Review Board (IRB) at King Saud University in Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
ESM 1
(DOCX 361 kb)
Rights and permissions
About this article
Cite this article
Okla, M., Zaher, W., Alfayez, M. et al. Inhibitory Effects of Toll-Like Receptor 4, NLRP3 Inflammasome, and Interleukin-1β on White Adipocyte Browning. Inflammation 41, 626–642 (2018). https://doi.org/10.1007/s10753-017-0718-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0718-y