Abstract
The α7 nicotinic acetylcholine receptor (α7-nAChR) is associated with inflammation, re-epithelialization, and angiogenesis in wound healing process. A recent study demonstrated that PNU-282987, a selective agonist of α7-nAChR, accelerates the repair of diabetic excisional wounds. Whether α7-nAChR activation promotes non-diabetic wounds healing is unknown. The aim of this study was to evaluate the effects of α7-nAChR activation on non-diabetic wound healing. The effects were evaluated in two wound models. In the first model, the wound was covered with a semi-permeable transparent dressing. In the second model, the wound was left uncovered. In both models, the mice were randomly assigned to two treatment groups: saline or PNU282987 (25 mice in each group). In covered wounds, we found that α7-nAChR activation inhibited re-epithelialization, angiogenesis, and epithelial cells proliferation, promoted neo-epithelial detachment, and suppressed neutrophil infiltration and the expression of interleukin-6 (IL-6) and vascular endothelial growth factor (VEGF). However, in uncovered wounds, we observed that α7-nAChR activation promoted re-epithelialization and angiogenesis, inhibited neutrophil infiltration and the expression of high mobility group box (HMGB)-1, epidermal growth factor (EGF), and VEGF. In conclusion, this data demonstrated that α7-nAChR activation inhibited wound healing in covered wounds but played an opposite role in uncovered wounds. The opposite effect might be primarily due to inhibition of inflammation.
Similar content being viewed by others
INTRODUCTION
Injury to the skin initiates a cascade of events including inflammation, new tissue formation, and tissue remodeling, which finally leads to at least partial reconstruction of the wounded area [1,2,3]. Nicotinic acetylcholine receptors are composed of different subunits, i.e. α1–α10, β1–β4, δ, γ, and ε, which combine to form pharmacologically distinct pentameric ion channels [4, 5]. The alpha7 nicotinic acetylcholine receptor (α7-nAChR), the homopentamer formed by α7 subunits, is an ion channel mediating the influx of Ca2+ and the protein kinase signaling cascades [6].
It has been reported that α7-nAChR is detected in keratinocytes, mature sebocytes, myoepithelial cells, endothelial cells, and macrophages [7,8,9,10]. In our previous study, a small number of polymorphonuclear cells, a large number of mononuclear cells, endothelial-like cells of regenerated vessels and neo-epidermis have been shown positive reaction for α7-nAChR in the wound zones [11].
α7-nAChR is associated with inflammation, re-epithelialization, and angiogenesis in wound healing. Soon after wounding, inflammatory cells invade the wound tissue. Neutrophils, monocytes, and lymphocytes execute defense functions and initiate the proliferative phase of wound repair [3]. α7-nAChR is an essential component of the cholinergic anti-inflammatory pathway [4, 9, 10]. In the new tissue formation phase, the epithelial cells near the wound edge become mobilized to migrate and proliferate to cover the denuded area [3]. These processes restore the epithelial surface and the barrier function of the skin [12,13,14]. α7-nAChR promotes keratinocyte directional migration but plays an opposite role if the receptor is overstimulated. Furthermore, α7-nAChR mediates the balance between keratinocyte proliferation and terminal differentiation [6, 7, 15,16,17,18,19,20]. Concurrent with re-epithelialization, massive angiogenesis leads to the formation of new blood vessels, facilitating the formation of granulation tissue [3]. α7-nAChR plays a pivotal role in physiological as well as pathological angiogenesis [4, 8, 21,22,23,24,25,26,27]. Nicotine, a non-selective agonist of α7-nAChR, was demonstrated to accelerate wound healing by promoting angiogenesis in a diabetic wound model [28].
In previous studies, PNU-282987 or nicotine was demonstrated to promote the healing process of diabetic wounds covered with transparent dressings [28, 29]. Whether α7-nAChR activation promotes non-diabetic wounds healing is unknown. This study aimed to evaluate the effects of α7-nAChR on non-diabetic wound healing.
MATERIALS AND METHODS
Reagents
The following reagents, including antibodies and ELISA kits, were obtained commercially: PNU282987 (P6499, Sigma-Aldrich, Shanghai, China), rabbit anti-MPO polyclonal antibody (pAb) (22225-1-AP, Proteintech, Wuhan, China), rabbit anti-F4/80 pAb (18705-1-AP, Proteintech, Wuhan, China), and rabbit anti-PCNA pAb (10205–2-AP, Proteintech, Wuhan, China), EGF ELISA kit (CSB-E08028m, CUSABIO, Wuhan, China), KGF ELISA kit (CSB-E13046m, CUSABIO, Wuhan, China), VEGF ELISA kit (CSB-E04756m, CUSABIO, Wuhan, China), bFGF ELISA kit (CSB-E08001m, CUSABIO, Wuhan, China), IL-6 ELISA kit (CSB-E04639m, CUSABIO, Wuhan, China), IL-1βELISA kit (CSB-E08053m, CUSABIO, Wuhan, China), TNF-αELISA kit (CSB-E04741m, CUSABIO, Wuhan, China), and HMGB1 ELISA kit (CSB-E08225m, CUSABIO, Wuhan, China) .
Animals and Wound Models
Eight-week-old healthy male BALB/c mice (n = 100, 18–26 g) were utilized. All mice had free access to tap water and rodent chow and were housed individually in a temperature-controlled animal facility with a 12-h light/dark cycle. Experiments conformed to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health published no.86-23, revised 1985) and the Guidelines for the Care and Use of Laboratory Animals of China Medical University.
Covered wound model and treatment groups: mice were anesthetized by intraperitoneal injection with sodium pentobarbital. Hair removal and sterilization were performed according to routine methods. Two 4 mm-diameter full-thickness wounds were made symmetrically with a sterile corneal trephine blade on the dorsal skin of each mouse and covered with a semi-permeable transparent dressing (Tegaderm, 3M Health Care, St. Paul, MN, USA). The forelimbs and necks of the mouse were fixed with Kenisio tape to reduce bites. All animals were placed on a heating pad until they fully recovered from anesthesia. Twenty five mice were assigned to either of two treatment groups: saline or PNU282987. Physiological saline or PNU282987 solution (0.1 mg/ml) was administrated daily, intraperitoneally in the same volume (10 ml/kg) from 10 min before wounding to 9 days after wounding. The dose of PNU282987 (1.0 mg/kg) was based on prior reports [29, 30]. Animals were euthanized at 1, 3, 5, 7, and 10 days after wounding (five animals/treatment groups per each sacrifice interval). Unilateral specimens were harvested and fixed with formalin and paraffin-embedded. The contralateral specimens (diameter of 8 mm) were harvested for enzyme linked immunosorbent assay (ELISA).
Uncovered wound model and treatment groups: as described above, two 4 mm diameter, full-thickness dermal excisional wounds were made on the dorsal skin of each mouse. The wounds were left uncovered, otherwise, all processes and treatment groups were the same as described above for the covered wound model.
Hematoxylin and Eosin Staining and Morphometric Analysis
The specimens were sectioned axially in the central area of the wound. Five-micrometer sections were prepared and stained with hematoxylin and eosin. The sections were scanned using an Aperio Digital Pathology Slide Scanner (Leica SCN400) at × 40 magnification. The slides were observed and measured using Aperio ImageScope software (version 12.2.2.2.5015). The neo-epidermis length (NL) was measured from the outermost hair root of the marginal skin to the margin of the neo-epidermis. The wound length (WL) was measured between two outermost hair roots of the marginal skin. The percentage of wound closure was calculated by NL/WL. The luminal structures containing red blood cells were defined as capillaries; the number of newly formed capillaries was counted in the wound bed extending to 500 μm outside the margin of wound in the slides by two pathologists in a blinded manner.
Immunohistochemical Staining and Morphometric Analysis
Immunohistochemical staining was performed for myeloperoxidase (MPO), F4/80, and proliferating cell nuclear antigen (PCNA). Color visualization was performed using SPlink Detection Kits (ZSGB-BIO, Beijing, China) according to the manufacturer’s instruction. Sections were routinely counterstained with hematoxylin. For negative control, sections were immunostained without the primary antibodies, which were replaced with normal IgG from the same species or PBS. No false-positive reaction was detected in the sections. Quantification of MPO+, F4/80+ and PCNA+ cells in the wound bed was performed using Image-Pro Plus software (version 6.0) with a set threshold level.
ELISA
The other specimens were homogenized in 10 volumes of PBS, and then homogenates were centrifuged three times at 12,000×g for 30 min at 4 °C. The levels of epidermal growth factor (EGF), keratinocyte growth factor (KGF), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), interleukin (IL)-6, IL-1β, tumor necrosis factor (TNF)-α, and high mobility group box (HMGB)-1 were measured in tissue extracts using commercially available ELISA kits according to the manufacturer’s instructions. The results were averaged and expressed as picograms per milligram of tissue.
Statistical Analysis
Data were analyzed using GraphPad Prism 5.0. The two-way ANOVA was used for data analysis between uncovered and covered groups. A two-tailed unpaired Student’s t test was used to analyze differences in covered groups as well as uncovered groups. The difference associated with P < 0.05 was considered statistically significant.
RESULTS
α7-nAChR Activation Effect on Re-epithelialization, Epithelial Cells Proliferation, Neo-Epithelial Detachment, EGF, and KGF Expression in Covered Wounds
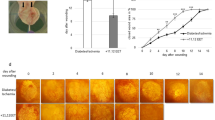
We evaluated the percentage of wound closure, the number of proliferating epithelial cells, and the levels of EGF and KGF in covered wounds. α7-nAChR activation inhibited re-epithelialization but insignificantly influenced the levels of EGF and KGF in covered wounds. Seven days after injury and thereafter, the percentage of wound closure in mice treated with PNU-282987 was consistently lower than in mice treated with saline (Fig. 1a, b). Compared with mice treated with saline, the wounds in mice treated with PNU-282987 had less proliferating epithelial cells at 7 days after injury (Fig. 1c, d). Ten days after injury, four wounds had neo-epithelial detachment in mice treated with PNU-282987 but detachment was not observed in mice treated with saline (Fig. 1e). From days 1 to 10 after wounding, the levels of EGF and KGF were not influenced significantly by PNU-282987 (Fig. 1f, g).
Re-epithelialization in covered wounds. The neo-epidermis in covered wounds (HE staining) 7d after wounding is shown (a). The two outer most vertical black arrows indicate the wound margins, and the distance between them is defined as the wound length (WL). Two middle vertical black arrows show the internal margins of neo-epidermis. The horizontal arrows indicate the neo-formed epithelium length (NL, I + II). The percentage of wound closure (NL/WL) in covered wounds is shown (b). Data are mean ± SE. Asterisk, P < 0.05. Immunohistochemical staining with PCNA antibody (c) and the number of proliferation keratinocytes (d) in covered wounds 7d after wounding are shown. Data are mean ± SD. Asterisk, P < 0.05. In covered wounds, the neo-formed epithelium was attached to the basal tissue in mice treated with saline (upper most picture), and detached from the basal tissue in mice treated with PNU-282987 (middle and bottom pictures) at 10 days after wounding (e). Black arrowheads indicate the detached neo-epidermis or naked basal tissue. The expression of EGF (f) and KGF (g) in covered wounds is shown.
α7-nAChR Activation Effect on Angiogenesis, VEGF, and bFGF Expression in Covered Wounds
We evaluated the number of capillaries and the levels of bFGF and VEGF in covered wounds. α7-nAChR activation inhibited angiogenesis and the level of VEGF but insignificantly influenced the levels of bFGF in covered wounds. Three days after injury, the number of capillaries in mice treated with PNU-282987 was lower than in mice treated with saline (Fig. 2a, b). From days 1 to 10 after wounding, the levels of bFGF were not influenced significantly by PNU-282987 (Fig. 2c). Seven days after injury, the level of VEGF in mice treated with PNU-282987 was lower than in mice treated with saline (Fig. 2d).
α7-nAChR Activation Effect on Re-epithelialization, Epithelial Cells Proliferation, EGF and KGF Expression in Uncovered Wounds
We evaluated the same parameters in the uncovered wound model. α7-nAChR activation promoted re-epithelialization and inhibited the level of EGF in uncovered wounds. Seven days after injury, re-epithelialization was complete in mice treated with PNU-282987 (Fig. 3a, b) although the numbers of proliferating epithelial cells were similar in mice treated with PNU-282987 or saline (Fig. 3c, d). Ten days after wounding, the level of EGF in mice treated with PNU-282987 was lower than in mice treated with saline (Fig. 3e). From days 1 to 10 after wounding, the level of KGF was not influenced by PNU-282987 (Fig. 3f).
Re-epithelialization in uncovered wounds. The neo-epidermis in uncovered wounds (HE staining) 7 days after wounding is shown (a). The percentage of wound closure (NL/WL) in uncovered wounds is shown (b). Data are mean ± SE. Asterisk, P < 0.05. Immunohistochemical staining with PCNA antibody (c) and the number of proliferation keratinocytes (d) in uncovered wounds 7 days after wounding are shown. Data are mean ± SD. The expression of EGF (e) and KGF (f) in uncovered wounds is shown. Data are mean ± SD. Double asterisk, P < 0.01.
α7-nAChR Activation Effect on Angiogenesis, VEGF, and bFGF Expression in Uncovered Wounds
α7-nAChR activation promoted angiogenesis and inhibited the level of VEGF in uncovered wounds. Three days after injury and thereafter, the number of capillaries in mice treated with PNU-282987 was higher than in mice treated with saline (Fig. 4a, b). From days 1 to 10 after wounding, the level of bFGF was not influenced significantly by PNU-282987 (Fig. 4c). Five and 10 days after injury, the level of VEGF in mice treated with PNU-282987 was lower than in mice treated with saline (Fig. 4d).
Angiogenesis in uncovered wounds. The capillaries in uncovered wounds (HE staining) are shown (a). Black arrowheads indicate the capillaries. The number of capillaries (b), the expression of bFGF (c) and VEGF (d) in uncovered wounds are shown. Data are mean ± SD. Asterisk, P < 0.05; double asterisk, P < 0.01.
Effect of Dressing on Re-epithelialization and Angiogenesis
The dressing inhibited re-epithelialization and angiogenesis. Three days after injury and thereafter, the percentage of wound closure and the number of capillaries in covered wounds were lower than those in uncovered wounds (Fig. 5a, b).
Effect of α7-nAChR Activation and Dressing on Inflammation
We compared the number of infiltrating neutrophils and macrophages, the levels of IL-1β, IL-6, HMGB1, and TNF-α both in covered and uncovered wounds. α7-nAChR activation inhibited neutrophil infiltration in covered and uncovered wounds. From days 3 to 5, the number of neutrophils in mice treated with PNU-282987 was lower than in mice treated with saline in uncovered wounds. Three days after injury, the number of neutrophils in mice treated with PNU-282987 was lower than in mice treated with saline in covered wounds. The dressing inhibited neutrophil infiltration greatly at the early-stage and promoted neutrophil infiltration at mid-stage healing. From days 1 to 3, the number of neutrophils in covered groups was less than in uncovered groups (Fig. 6a, b). Five days after wounding, the number of neutrophils in covered groups was higher than in uncovered groups (Fig. 6b). α7-nAChR activation promoted early-stage macrophage infiltration in covered wounds. One day after wounding, the number of macrophages in mice treated with PNU-282987 was higher than in mice treated with saline in covered wounds. The dressing inhibited all-stage macrophage infiltration. From days 1 to 10 after injury, the number of macrophages in covered groups was less than in uncovered groups (Fig. 6a, c). α7-nAChR activation and the dressing inhibited the expression of pro-inflammatory cytokines. Three days after injury, the expression of IL-6 and HMGB1 in mice treated with PNU-282987 was lower than in mice treated with saline in covered and uncovered wounds, respectively (Fig. 6e, f). One day after wounding, the expression of IL-6 in covered groups was less than in uncovered groups (Fig. 6e). Five days after wounding, the expression of HMGB1 in covered groups was less than in uncovered groups (Fig. 6f). From day 1 to 10 after wounding, the levels of IL-1β and TNF-α were not influenced significantly by PNU-282987 and dressing (Fig. 6d, g).
Effects of α7-nAChR activation and dressing on inflammation. Immunohistochemical staining with MPO and F4/80 antibody (a), the number of neutrophils (b), macrophages (c), and the expression of IL-1β (d), IL-6 (e), HMGB1 (f), and TNF-α (g) in covered and uncovered wounds are shown. Data are mean ± SD. Asterisk, P < 0.05; double asterisk, P < 0.01; triple asterisk, P < 0.001.
DISCUSSION
The results demonstrated an opposite effect of α7-nAChR activation on wound healing. α7-nAChR activation inhibited re-epithelialization and angiogenesis in covered wounds and played an opposite role in uncovered wounds. The opposite effect might be primarily due to differing degrees of inhibition of inflammation.
To investigate whether α7-nAChR activation promotes wound healing, we evaluated re-epithelialization, angiogenesis, epithelial cells proliferation, and expression of EGF, KGF, bFGF, and VEGF in covered wounds. Partly contrary to previous works [28, 29], we found that α7-nAChR activation inhibited re-epithelialization and angiogenesis. Others found that α7-nAChR promotes directional migration of keratinocytes [17, 19], and that overstimulation of the receptor could cause receptor desensitization and delay wound re-epithelialization [31]. We suspect that the administration mode of PNU-282987 in our experiment overstimulated α7-nAChR and contributed partly to the inhibition of re-epithelialization. Furthermore, a prior study showed that α7-nAChR knockout mice have a highly proliferative, thickened epidermis [16], and we found that α7-nAChR activation inhibited epithelial cell proliferation and promoted neo-epithelial detachment, suggesting that the inhibition of epithelial cells proliferation contributes partly to the delayed re-epithelialization. Previous reports demonstrated that nicotine, a non-selective agonist of α7-nAChR, promoted angiogenesis in non-diabetic wound models [32, 33] and selective stimulation of α7-nAChR resulted in a significant release of VEGF [8]. In covered wounds, we found opposite effects; α7-nAChR activation inhibited angiogenesis and VEGF expression, suggesting that other factors modify the inhibition of angiogenesis and VEGF expression by α7-nAChR activation.
Dressings are known to influence wound healing [34,35,36,37]. We doubted that the dressing may explain why our results differed from previous works, so we repeated the experiment in an uncovered wound model. To our surprise, we found that α7-nAChR activation promoted re-epithelization and angiogenesis in uncovered wounds, which is consistent with prior works showing that nicotine enhanced non-diabetic and diabetic wounds healing by promoting angiogenesis [28, 32, 33]. Although α7-nAChR activation was reported to promote keratinocyte migration [17, 19], and overstimulation of the receptor was suggested to delay wound re-epithelialization [31], we found that the same dosage and duration of PNU-282987 produced an opposite effect on epithelia cells migration in covered and uncovered wounds. In addition, our data demonstrated that the same dosage and duration of the agonist did not significantly influence epithelial cells proliferation and neo-epithelial detachment in uncovered wounds, which differed from its effect on covered wounds. The different effects of α7-nAChR activation on re-epithelialization in covered and uncovered wounds support the view that other pathways, besides directly affecting keratinocytes, might influence re-epithelialization. We further found that α7-nAChR activation promoted angiogenesis in uncovered wounds and played a contrary role in covered wounds, however, it inhibited VEGF expression in both covered and uncovered wounds, implying that other factors might influence angiogenesis.
Of note, α7-nAChR activation promotes wound healing in covered diabetic [29] and uncovered non-diabetic wounds, suggesting those wound healing processes might share a common mechanism. Skin wounds in adult animals have evolved into robust inflammation unfavorable to wound closure, whereas depletion of neutrophils accelerates wound closure [13, 38,39,40,41,42]. Similarly, intense inflammation is associated with diabetic wounds, while reduction of neutrophil infiltration is associated with enhanced healing [39, 43,44,45,46,47]. We found that α7-nAChR activation inhibited the infiltration of neutrophils and the expression of HMGB1 in uncovered wounds and a previous work demonstrated that the neutrophil infiltration in the skin caused by UV irradiation was dependent on HMGB1 [48]. Thus, it is reasonable to believe that the inhibition of neutrophil infiltration and HMGB1 expression contributed, at least in part, to the accelerated wound closure we observed in uncovered wounds of mice in the α7-nAChR activation group.
Inflammation is a double-edged sword; although excessive inflammation might inhibit tissue repair, adequate inflammation is critical for successful wound healing [41, 42, 49]. Dressings have been reported to create a clean, sterile environment and inhibit inflammatory response [34,35,36,37]. Our data confirmed that the dressing inhibited the infiltration of neutrophils and macrophages as well as the expression of IL-6 and HMGB1. Moreover, the dressing inhibited re-epithelialization and angiogenesis significantly, suggesting that anti-inflammatory effect of the dressing could inhibit wound healing. Furthermore, we found that, in the context of the anti-inflammatory effects of the dressing, α7-nAChR activation suppressed neutrophils infiltration and IL-6 expression in covered wounds. Re-epithelialization and granulation tissue formation were demonstrated to be impaired in IL-6 knockout animals [3], and keratinocyte nAChR activation was shown to dampen Toll-like receptor 2 mediated keratinocyte migration, which is restored by an α7-selective nAChR antagonist [50]. Therefore, we reasoned that the “over-inhibition” of inflammatory response by α7-nAChR activation suppressed re-epithelialization in our covered wound model. On the other hand, angiogenesis and inflammation are co-dependent processes, and nicotine was demonstrated to stimulate angiogenesis in the setting of inflammation [8, 22, 24, 26], suggesting that the “over-inhibition” of inflammation might reverse the angiogenic effects of α7-nAChR activation in covered wounds.
In conclusion, we provide evidence that α7-nAChR activation inhibited covered wound healing and played an opposite role in uncovered wounds. The extent of anti-inflammatory effect might explain this apparent contradiction; moderate anti-inflammation would accelerate wound healing, but over-inhibition of inflammation would delay wound repair.
References
Singer, A.J., and R.A. Clark. 1999. Cutaneous wound healing. New England Journal of Medicine 341: 738–746.
Gurtner, G.C., S. Werner, Y. Barrandon, and M.T. Longaker. 2008. Wound repair and regeneration. Nature 453: 314–321.
Werner, S., and R. Grose. 2003. Regulation of wound healing by growth factors and cytokines. Physiological Reviews 83: 835–870.
Pillai, S., and S. Chellappan. 2012. Alpha7 nicotinic acetylcholine receptor subunit in angiogenesis and epithelial to mesenchymal transition. Current Drug Targets 13: 671–679.
Kurzen, H., I. Wessler, C. Kirkpatrick, K. Kawashima, and S. Grando. 2007. The non-neuronal cholinergic system of human skin. Hormone and Metabolic Research 39: 125–135.
Chernyavsky, A.I., J. Arredondo, J. Qian, V. Galitovskiy, and S.A. Grando. 2009. Coupling of ionic events to protein kinase signaling cascades upon activation of α7 nicotinic receptor: Cooperative regulation of 2-integrin expression and Rho Kinase activity. Journal of Biological Chemistry 284: 22140–22148.
Grando, S.A., M.R. Pittelkow, and K.U. Schallreuter. 2006. Adrenergic and cholinergic control in the biology of epidermis: physiological and clinical significance. Journal of Investigative Dermatology 126: 1948–1965.
Heeschen, C., M. Weis, A. Aicher, S. Dimmeler, and J.P. Cooke. 2002. A novel angiogenic pathway mediated by non-neuronal nicotinic acetylcholine receptors. Journal of Clinical Investigation 110: 527–536.
Gallowitsch-Puerta, M. 2005. Immunologic role of the cholinergic anti-inflammatory pathway and the nicotinic acetylcholine α7 receptor. Annals of the New York Academy of Sciences 1062: 209–219.
Wang, H., M. Yu, M. Ochani, C.A. Amella, M. Tanovic, S. Susarla, J.H. Li, H. Wang, H. Yang, L. Ulloa, Y. Al-Abed, C.J. Czura, and K.J. Tracey. 2003. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421: 384–388.
Fan, Y., T. Yu, T. Wang, W. Liu, R. Zhao, S. Zhang, W. Ma, J. Zheng, and D.W. Guan. 2011. Nicotinic acetylcholine receptor alpha7 subunit is time-dependently expressed in distinct cell types during skin wound healing in mice. Histochemistry and Cell Biology 135: 375–387.
Jacinto, A., A. Martinez-Arias, and P. Martin. 2001. Mechanisms of epithelial fusion and repair. Nature Cell Biology 3: E117–E123.
Dovi, J.V., A.M. Szpaderska, and L.A. DiPietro. 2004. Neutrophil function in the healing wound: adding insult to injury? Thrombosis and Haemostasis 92: 275–280.
Fang, Y., and K.K.H. Svoboda. 2005. Nicotine inhibits human gingival fibroblast migration via modulation of Rac signalling pathways. Journal of Clinical Periodontology 32: 1200–1207.
Kurzen, H., C. Henrich, D. Booken, N. Poenitz, A. Gratchev, C. Klemke, M. Engstner, S. Goerdt, and N. Maas-Szabowski. 2006. Functional characterization of the epidermal cholinergic system in vitro. Journal of Investigative Dermatology 126: 2458–2472.
Arredondo, J. 2002. Central role of alpha7 nicotinic receptor in differentiation of the stratified squamous epithelium. The Journal of Cell Biology 159: 325–336.
Chernyavsky, A.I. 2004. Differential regulation of keratinocyte chemokinesis and chemotaxis through distinct nicotinic receptor subtypes. Journal of Cell Science 117: 5665–5679.
Arredondo, J., V.T. Nguyen, A.I. Chernyavsky, D. Bercovich, A. Orr-Urtreger, D.E. Vetter, and S.A. Grando. 2003. Functional role of alpha7 nicotinic receptor in physiological control of cutaneous homeostasis. Life Sciences 72: 2063–2067.
Chernyavsky, A.I., J. Arredondo, E. Karlsson, I. Wessler, and S.A. Grando. 2005. The Ras/Raf-1/MEK1/ERK signaling pathway coupled to integrin expression mediates cholinergic regulation of keratinocyte directional migration. Journal of Biological Chemistry 280: 39220–39228.
Costa, F., and R. Soares. 2009. Nicotine: a pro-angiogenic factor. Life Sciences 84: 785–790.
Ng, M.K., J. Wu, E. Chang, B.Y. Wang, R. Katzenberg-Clark, A. Ishii-Watabe, and J.P. Cooke. 2007. A central role for nicotinic cholinergic regulation of growth factor-induced endothelial cell migration. Arteriosclerosis, Thrombosis, and Vascular Biology 27: 106–112.
Heeschen, C., E. Chang, A. Aicher, and J.P. Cooke. 2006. Endothelial progenitor cells participate in nicotine-mediated angiogenesis. Journal of the American College of Cardiology 48: 2553–2560.
Park, Y.J., T. Lee, J. Ha, I.M. Jung, J.K. Chung, and S.J. Kim. 2008. Effect of nicotine on human umbilical vein endothelial cells (HUVECs) migration and angiogenesis. Vascular Pharmacology 49: 32–36.
Heeschen, C., J.J. Jang, M. Weis, A. Pathak, S. Kaji, R.S. Hu, P.S. Tsao, F.L. Johnson, and J.P. Cooke. 2001. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nature Medicine 7: 833–839.
Egleton, R.D., K.C. Brown, and P. Dasgupta. 2009. Angiogenic activity of nicotinic acetylcholine receptors: implications in tobacco-related vascular diseases. Pharmacology & Therapeutics 121: 205–223.
Pena, V.B., I.C. Bonini, S.S. Antollini, T. Kobayashi, and F.J. Barrantes. 2011. Alpha 7-type acetylcholine receptor localization and its modulation by nicotine and cholesterol in vascular endothelial cells. Journal of Cellular Biochemistry 112: 3276–3288.
Li, X., and H. Wang. 2006. Non-neuronal nicotinic alpha 7 receptor, a new endothelial target for revascularization. Life Sciences 78: 1863–1870.
Jacobi, J., J.J. Jang, U. Sundram, H. Dayoub, L.F. Fajardo, and J.P. Cooke. 2002. Nicotine accelerates angiogenesis and wound healing in genetically diabetic mice. The American Journal of Pathology 161: 97–104.
Dong, M.W., M. Li, J. Chen, T.T. Fu, K.Z. Lin, G.H. Ye, J.G. Han, X.P. Feng, X.B. Li, L.S. Yu, and Y.Y. Fan. 2016. Activation of alpha7nAChR promotes diabetic wound healing by suppressing AGE-induced TNF-alpha production. Inflammation 39: 687–699.
Su, X., J.W. Lee, Z.A. Matthay, G. Mednick, T. Uchida, X. Fang, N. Gupta, and M.A. Matthay. 2007. Activation of the α7 nAChR reduces acid-induced acute lung injury in mice and rats. American Journal of Respiratory Cell and Molecular Biology 37: 186–192.
Grando, S.A. 2006. Cholinergic control of epidermal cohesion. Experimental Dermatology 15: 265–282.
Morimoto, N., S. Takemoto, T. Kawazoe, and S. Suzuki. 2008. Nicotine at a low concentration promotes wound healing. Journal of Surgical Research 145: 199–204.
Liem, P.H., N. Morimoto, R. Ito, K. Kawai, and S. Suzuki. 2013. Treating a collagen scaffold with a low concentration of nicotine promoted angiogenesis and wound healing. Journal of Surgical Research 182: 353–361.
Kloeters, O., C. Schierle, A. Tandara, and T.A. Mustoe. 2008. The use of a semiocclusive dressing reduces epidermal inflammatory cytokine expression and mitigates dermal proliferation and inflammation in a rat incisional model. Wound Repair and Regeneration 16: 568–575.
Dyson, M., S. Young, C.L. Pendle, D.F. Webster, and S.M. Lang. 1988. Comparison of the effects of moist and dry conditions on dermal repair. Journal of Investigative Dermatology 91: 434–439.
Hien, N.T., S.E. Prawer, and H.I. Katz. 1988. Facilitated wound healing using transparent film dressing following Mohs micrographic surgery. Archives of Dermatology 124: 903–906.
Park, S.A., L.B. Teixeira, V.K. Raghunathan, J. Covert, R.R. Dubielzig, R.R. Isseroff, M. Schurr, N.L. Abbott, J. McAnulty, and C.J. Murphy. 2014. Full-thickness splinted skin wound healing models in db/db and heterozygous mice: implications for wound healing impairment. Wound Repair and Regeneration 22: 368–380.
Eming, S.A., T. Krieg, and J.M. Davidson. 2007. Inflammation in wound repair: molecular and cellular mechanisms. Journal of Investigative Dermatology 127: 514–525.
Dovi, J.V., L.K. He, and L.A. DiPietro. 2003. Accelerated wound closure in neutrophil-depleted mice. Journal of Leukocyte Biology 73: 448–455.
Weiss, S.J. 1989. Tissue destruction by neutrophils. New England Journal of Medicine 320: 365–376.
Martin, P., D. D'Souza, J. Martin, R. Grose, L. Cooper, R. Maki, and S.R. McKercher. 2003. Wound healing in the PU.1 null mouse—tissue repair is not dependent on inflammatory cells. Current Biology 13: 1122–1128.
Stramer, B.M., R. Mori, and P. Martin. 2007. The inflammation-fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. Journal of Investigative Dermatology 127: 1009–1017.
Wong, S.L., M. Demers, K. Martinod, M. Gallant, Y. Wang, A.B. Goldfine, C.R. Kahn, and D.D. Wagner. 2015. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nature Medicine 21: 815–819.
Falanga, V. 2005. Wound healing and its impairment in the diabetic foot. Lancet 366: 1736–1743.
Lan, C.C., C.S. Wu, S.M. Huang, I.H. Wu, and G.S. Chen. 2013. High-glucose environment enhanced oxidative stress and increased interleukin-8 secretion from keratinocytes: new insights into impaired diabetic wound healing. Diabetes 62: 2530–2538.
Baltzis, D., I. Eleftheriadou, and A. Veves. 2014. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: new insights. Advances in Therapy 31: 817–836.
Xu, F., C. Zhang, and D.T. Graves. 2013. Abnormal cell responses and role of TNF-alpha in impaired diabetic wound healing. BioMed Research International 2013: 754802.
Leavy, O. 2014. Tumour immunology: inflaming tumour spread. Nature Reviews Immunology 14: 212.
Razani-Boroujerdi, S., S.P. Singh, C. Knall, F.F. Hahn, J.C. Peña-Philippides, R. Kalra, R.J. Langley, and M.L. Sopori. 2004. Chronic nicotine inhibits inflammation and promotes influenza infection. Cellular Immunology 230: 1–9.
Kishibe, M., T.M. Griffin, and K.A. Radek. 2015. Keratinocyte nicotinic acetylcholine receptor activation modulates early TLR2-mediated wound healing responses. International Immunopharmacology 29: 63–70.
Acknowledgments
This study was financially supported by grants from the National Natural Science Foundation of China (81273342, 81671862).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, JY., Jiang, SK., Wang, LL. et al. α7-nAChR Activation Has an Opposite Effect on Healing of Covered and Uncovered Wounds. Inflammation 41, 474–484 (2018). https://doi.org/10.1007/s10753-017-0703-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0703-5