Abstract
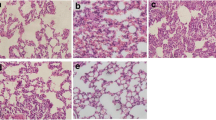
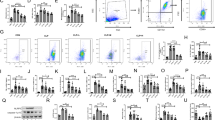
Acute lung injury (ALI) is mainly characterized by diffusive injuries to lung epithelium and increased permeability of alveolar-capillary membranes caused by various factors, which leads to pulmonary edema and pulmonary closure. Lipopolysaccharide (LPS), which is the main component of the cell wall of gram-negative bacteria, is one of the most important factors causing pulmonary infection and ALI. More and more reports have indicated that hydrogen sulfide (H2S) is closely correlated with ALI and has anti-inflammation function, while the specific mechanism needs further investigation. Cholecystokinin-octapeptide (CCK-8), which is an important endogenous functional fragment belonging to CCK family, participates in anti-inflammatory and anti-endotoxic shock (ES). Whether CCK-8 plays important roles in curing ALI also needs further investigation. Herein, we concluded that CCK-8 alleviated the ALI induced by LPS via regulating the catalytic activity of cystathionine γ-lyase (CSE) and the formation of H2S. This work provides new medicine-designed target for clinical doctor to prevent and cure ALI.




Similar content being viewed by others
Reference
Mouratis, M.A., et al. 2015. Autotaxin and Endotoxin-Induced Acute Lung Injury. PLoS One 10(7): e0133619.
Ware, L.B. 2006. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Seminars in Respiratory and Critical Care Medicine 27(4): 337–49.
Donahoe, M. 2011. Acute respiratory distress syndrome: A clinical review. Pulmonary Circulatory 1(2): 192–211.
Ragaller, M., and T. Richter. 2010. Acute lung injury and acute respiratory distress syndrome. Journal of Emergencies, Trauma and Shock 3(1): 43–51.
Elizabeth, R., and A.M.A.M. Johnson. 2010. Acute Lung Injury Epidemiology, Pathogenesis, and Treatment. Journal Aerosol Medicine Pulmonary Drug Delivery 23(4): 243–252.
Brown, L., et al. 2015. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nature Reviews Microbiology 13(10): 620–30.
Ramachandran, G. 2014. Gram-positive and gram-negative bacterial toxins in sepsis: a brief review. Virulence 5(1): 213–8.
Rittirsch, D., et al. 2008. Acute Lung Injury Induced by Lipopolysaccharide Is Independent of Complement Activation. The Journal of Immunology 180(11): 7664–7672.
Gu, H., et al. 2013. Necro-inflammatory response of pancreatic acinar cells in the pathogenesis of acute alcoholic pancreatitis. Cell Death & Disease 4, e816.
Li, S., et al. 2007. CCK-8 inhibits LPS-induced IL-1beta production in pulmonary interstitial macrophages by modulating PKA, p38, and NF-kappaB pathway. Shock 27(6): 678–86.
Li, S., Z. Ni, B. Cong, W. Gao, S. Xu, C. Wang, Y. Yao, C. Ma, and Y. Ling. 2007. Cholecystokinin plays a novel protective role in diabetic kidney through anti-inflammatory actions on macrophage anti-inflammatory effect of cholecystokinin. Shock 27(6): 678–686.
Romero, C. and M.A. Bosch. Effect of dimethylthiourea in phosphatidylcholine biosynthesis by rat lung during reversible endotoxic shock. (0300–8177 (Print)).
Huang, X.-L., Y.-L. Ling, Y.-Q. Ling, J.-L. Zhou, Y. Liu, and Q.-H. Wang. 2004. Heme oxygenase-1 in cholecystokinin-octapeptipe attenuated injury of pulmonary artery smooth muscle cells induced by lipopolysaccharide and its signal transduction mechanism. World Journal of Gastroenterol 10(12): 1789–1794.
Ling, Y.-L., X.-L. Huang, Y.-Q. Ling, J.-L. Zhou, Y. Liu, and Q.-H. Wang. 2004. Heme oxygenase-1 in cholecystokinin-octapeptipe attenuated injury of pulmonary artery smooth muscle cells induced by lipopolysaccharide and its signal transduction mechanism. World Journal of Gastroenterology 10(12): 1789–1794.
Guo, H., et al. 2014. Electroacupuncture Ameliorates Acute Lung Injury through Promoting Gastrointestinal Motility in Rats with Acute Pancreatitis. Evidence-Based Complementary and Alternative Medicine 2014: 1–8.
Bhatia, H.Z.A.M. 2009. Role of Hydrogen Sulfide in Acute Lung Injury and Acute Respiratory Distress Syndrome. The Open Critical Care Medicine Journal 2: 13–17.
Fu, M., et al. 2012. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proceedings of the National Academy of Sciences of the United States of America 109(8): 2943–8.
You, X.J., et al. 2011. Expression of cystathionine beta-synthase and cystathionine gamma-lyase in human pregnant myometrium and their roles in the control of uterine contractility. PLoS One 6(8): e23788.
Patel, P., et al. 2009. The endogenous production of hydrogen sulphide in intrauterine tissues. Reproductive Biology and Endocrinology 7: 10.
Madden, J.A., et al. 2012. Precursors and inhibitors of hydrogen sulfide synthesis affect acute hypoxic pulmonary vasoconstriction in the intact lung. Journal of Applied Physiologica (1985) 112(3): 411–8.
Li, T., et al. 2008. Regulatory effects of hydrogen sulfide on IL-6, IL-8 and IL-10 levels in the plasma and pulmonary tissue of rats with acute lung injury. Experimental Biology and Medicine (Maywood, NJ) 233(9): 1081–7.
Faller, S., K. Zimmermann, K.M. Strosing, H. Engelstaedter, H. Buerkle, R. Schmidt, S.G. Spassov, and A. Hoetzel. 2012. Inhaled hydrogen sulfide protects against lipopolysaccharide-induced acute lung injury in mice. Medicine Gas Research 12(26): 1–6.
Runmin Guo, K.W., J. Chen, L. Mo, X. Hua, D. Zheng, P. Chen, G. Chen, W. Xu, and J. Feng. 2013. Exogenous Hydrogen Sulfide Protects against Doxorubicin-Induced Inflammation and Cytotoxicity by Inhibiting p38MAPK/NFκB Pathway in H9c2 Cardiac Cells. Cellular Physiology and Biochemistry 32: 1668–1680.
Zhou, X.H., X. Huang, P. Wei, F.J. Tian, and Y.L. Ling. 2009. Role of hydrogen sulfidecystathionine-gamma-lyase system in acute lung injury induced by lipopolysaccharide in rats. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 21(4): 199–202.
Smith, K.M., et al., Prolonged partial liquid ventilation using conventional and high-frequency ventilatory techniques: gas exchange and lung pathology in an animal model of respiratory distress syndrome. (0090–3493 (Print)).
Szapiel, S.V., N. Elson, J.D. Fulmer, and G.W. Hunninghake. 1979. Crystal RG, Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. The American Review of Respiratory Disease 120(4): 893–899.
Chen, X., et al. 2014. Hydrogen sulfide reduces kidney injury due to urinary-derived sepsis by inhibiting NF-kappaB expression, decreasing TNF-alpha levels and increasing IL-10 levels. Experimental and Therapeutic Medicine 8(2): 464–470.
Huang, S., H. Li, and J. Ge. 2015. A cardioprotective insight of the cystathionine γ-lyase/hydrogen sulfide pathway. IJC Heart & Vasculature 7: 51–57.
Pan, L.L., et al. 2012. Role of cystathionine gamma-lyase/hydrogen sulfide pathway in cardiovascular disease: a novel therapeutic strategy? Antioxidants and Redox Signaling 17(1): 106–18.
Chiku, T., et al. 2009. H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. Journal of Biological Chemistry 284(17): 11601–12.
Leffler, C.W., et al. 2006. Carbon monoxide and hydrogen sulfide: gaseous messengers in cerebrovascular circulation. Journal of Applied Physiologica (1985) 100(3): 1065–76.
Ling, Y.-L., A.-H. Meng, X.-P. Zhang, and J.-L. Zhang. 2002. Anti-inflammatory effect of cholecystokinin and its signal transduction mechanism in endotoxic shock rat. World Journal of Gastroenterology 8(4): 712–717.
Verspohl Ej Fau - Wunderle, G., et al., Proglumide (gastrin and cholecystokinin receptor antagonist) inhibits insulin secretion in vitro. (0028–1298 (Print)).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tian, F., Ling, Y., Chen, Y. et al. Effects of CCK-8 and Cystathionine γ-Lyase/Hydrogen Sulfide System on Acute Lung Injury in Rats. Inflammation 40, 174–183 (2017). https://doi.org/10.1007/s10753-016-0466-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0466-4