Abstract
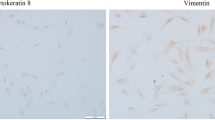
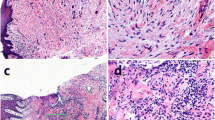
Human gingival fibroblasts (HGFs) are the major constituents of the gingival tissues responsible for the synthesis and degradation of the connective tissue while actively participating in immune reactions and inflammation. The aim of this study was to test the impact of lipopolysaccharide (LPS) from Porphyromonas gingivalis (P. gingivalis) on human gingival fibroblasts. Human gingival fibroblasts were treated with different P. gingivalis LPS concentrations. Cell survival rate was evaluated with 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) after 24 h. Cell proliferation was determined by counting cells on days 3 and 12. Expression of matrix metalloproteinases (MMPs), tissue inhibitors of MMPs (TIMPs), and pro-inflammatory cytokine transcripts in HGFs was determined by quantitative PCR (Q-PCR) analysis on days 3 and 8. P. gingivalis LPS decreased cell proliferation on day 3 (p < 0.05) compared to the control group without significantly impacting the cell survival (p > 0.05).The experiments showed that P. gingivalis LPS dose-dependently and differentially modulated the expression of MMP-1, 2, and 3 and TIMP-1 and 2 on days 3 and 8. TIMP-1 expression was significantly induced in P. gingivalis LPS-treated cells while TIMP-2 was increased in response to 10 and 30 ng/ml of LPS on day 3. P. gingivalis LPS induced up-regulation of MMP-1/TIMP-1 ratio on day 3 and increased MMP-2/TIMP-2 ratio on day 8 dose-dependently. Expression of interleukin (IL)-6 and IL-8 was stimulated at higher concentrations (1000 and 3000 ng/ml) of LPS. These findings demonstrate that P. gingivalis LPS suppresses cell proliferation and leads to increased pro-inflammatory changes in HGFs, suggesting that P. gingivalis LPS-induced modification of phenotypic and inflammatory characteristics in HGF could potentially be a pathogenic mechanism underlying the tissue destruction.





Similar content being viewed by others
References
Garlet, G.P. 2010. Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. Journal of Dental Research 89(12): 1349–1363.
Chi, X.P., X.Y. Ouyang, and Y.X. Wang. 2014. Hydrogen sulfide synergistically upregulates Porphyromonas gingivalis lipopolysaccharide-induced expression of IL-6 and IL-8 via NF-κB signalling in periodontal fibroblasts. Archives of Oral Biology 59(9): 954–961.
Li, X., X. Wang, M. Zheng, and Q.X. Luan. 2016. Mitochondrial reactive oxygen species mediate the lipopolysaccharide-induced pro-inflammatory response in human gingival fibroblasts. Experiment Cell Research. doi:10.1016/j.yexcr.2016.08.007.
Rizzo, A., R. Paolillo, L. Guida, M. Annunziata, N. Bevilacqua, and M.A. Tufano. 2010. Effect of metronidazole and modulation of cytokine production on human periodontal ligament cells. International Immunopharmacology 10: 744–750.
Baek, K.J., S. Ji, Y.C. Kim, and Y. Choi. 2015. Association of the invasion ability of Porphyromonas gingivalis with the severity of periodontitis. Virulence 6(3): 274–281.
Ardila, C.M., M. Olarte-Sossa, and A.A. Ariza-Garcés. 2015. Association between the presence ofTreponema denticola and reduced levels of antiatherogenic high density lipoprotein in periodontitis. Quintessence International 46(3): 207–215.
Herath, T.D., R.P. Darveau, C.J. Seneviratne, C.Y. Wang, Y. Wang, and L. Jin. 2016. HeterogeneousPorphyromonas gingivalis LPS modulates immuno-inflammatory response, antioxidant defense and cytoskeletal dynamics in human gingival fibroblasts. Science Reports. doi:10.1038/srep29829.
Morandini, A.C., C.R. Sipert, T.H. Gasparoto, S.L. Greghi, E. Passanezi, M.L. Rezende, et al. 2010. Differential production of macrophage inflammatory protein-1alpha, stromal-derived factor-1, and IL-6 by human cultured periodontal ligament and gingival fibroblasts challenged with lipopolysaccharide from P. gingivalis. Journal of Periodontology 81: 310–317.
Ding, P.H., and L.J. Jin. 2014. The role of lipopolysaccharide-binding protein in innate immunity: a revisit and its relevance to oral/periodontal health. Journal of Periodontal Research 49(1): 1–9.
Ara, T., K. Kurata, K. Hirai, T. Uchihashi, T. Uematsu, and Y. Imamura. 2009. Human gingival fibroblasts are critical in sustaining inflammation in periodontal disease. Journal of Periodontal Research 44: 21–27.
Morimoto, Y., K. Kikuchi, and T. Ito. 2009. MK615 attenuates Porphyromonas gingivalis lipopolysaccharide-induced pro-inflammatory cytokine release via MAPK inactivation in murine macrophage-like RAW264.7 cells. Biochemical and Biophysical Research Communications 389: 90–94.
Herath, T.D., Y. Wang, C.J. Seneviratne, R.P. Darveau, C.Y. Wang, and L. Jin. 2013. The expression and regulation of matrix metalloproteinase-3 is critically modulated by Porphyromonas gingivalis lipopolysaccharide with heterogeneous lipid A structures in human gingival fibroblasts. BMC Microbiology 13: 73.
Scheres, N., M.L. Laine, T.J. de Vries, V. Everts, and A.J. van Winkelhoff. 2010. Gingival and periodontal ligament fibroblasts differ in their inflammatory response to viable Porphyromonas gingivalis. Journal of Periodontal Research 45: 262–270.
Into, T., M. Inomata, K. Shibata, and Y. Murakami. 2010. Effect of the antimicrobial peptide LL-37 on Toll-like receptors 2-, 3- and 4-triggered expression of IL-6, IL-8 and CXCL10 in human gingival fibroblasts. Cellular Immunology 264: 104–109.
Herath, T.D., Y. Wang, C.J. Seneviratne, Q. Lu, R.P. Darveau, C.Y. Wang, and L. Jin. 2011. Porphyromonas gingivalis lipopolysaccharide lipid A heterogeneity differentially modulates the expression of IL-6 and IL-8 in human gingival fibroblasts. Journal of Clinical Periodontology 38(8): 694–701.
Genco, C.A., C.W. Cutler, D. Kapczynski, K. Maloney, and R.R. Arnold. 1991. A novel mouse model to study the virulence of and host response to Porphyromonas (Bacteroides) gingivalis. Infection and Immunity 59: 1255–1263.
Livak, K.J., and T.D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408.
Derradjia, A., H. Alanazi, H.J. Park, R. Djeribi, A. Semlali, and M. Rouabhia. 2016. α-tocopherol decreases interleukin-1β and -6 and increases human β-defensin-1 and -2 secretion in human gingival fibroblasts stimulated with Porphyromonas gingivalis lipopolysaccharide. Journal of Periodontal Research 51(3): 295–303.
Konermann, A., D. Stabenow, P.A. Knolle, S.A. Held, J. Deschner, and A. Jäger. 2012. Regulatory role of periodontal ligament fibroblasts for innate immune cell function and differentiation. Innate Immunology 18(5): 745–752.
Yamaji, Y., T. Kubota, K. Sasaguri, S. Sato, Y. Suzuki, H. Kumada, et al. 1995. Inflammatory cytokine gene expression in human periodontal ligament fibroblasts stimulated with bacterial lipopolysaccharides. Infect Immunology 63: 3576–3581.
Pellegrini, G., G. Rasperini, G. Pagni, W.V. Giannobile, S. Milani, F. Musto, and C. Dellavia. 2016. Local wound healing biomarkers for real-time assessment of periodontal regeneration: pilot study. Journal of Periodontal Research. doi:10.1111/jre.12403.
Kuo, P.J., H.L. Lin, C.Y. Lin, Y.T. Chin, H.P. Tu, T.M. Lai, et al. 2016. Crosstalk between human monocytic U937 cells and gingival fibroblasts in co-culturally enhanced matrix metalloproteinase-2 expression. Journal of Periodontology 13: 1–19.
Sapna, G., S. Gokul, and K. Bagri-Manjrekar. 2014. Matrix metalloproteinases and periodontal diseases. Oral Diseases 20(6): 538–50.
Zeldich, E., R. Koren, M. Dard, E. Weinberg, M. Weinreb, and C.E. Nemcovsky. 2010. Enamel matrix derivative induces the expression of tissue inhibitor of matrix metalloproteinase-3 in human gingival fibroblasts via extracellular signal-regulated kinase. Journal of Periodontal Research 45(2): 200–206.
Hannas, A.R., J.C. Pereira, J.M. Granjeiro, and L. Tjäderhane. 2007. The role of matrix metalloproteinases in the oral environment. Acta Odontologica Scandinavica 65(1): 1–13.
Koka, S., and R.A. Reinhardt. 1997. Periodontal pathogen-related stimulation indicates unique phenotype of primary cultured human fibroblasts from gingiva and periodontal ligament: implications for oral health disease. Journal of Prosthetic Dentistry 77(2): 191–196.
Pattamapun, K., S. Tiranathanagul, T. Yongchaitrakul, J. Kuwatanasuchat, and P. Pavasant. 2003. Activation of MMP-2 by Porphyromonas gingivalis in human periodontal ligament cells. Journal of Periodontal Research 38: 115–121.
Beklen, A., M. Ainola, M. Hukkanen, C. Gürgan, T. Sorsa, and Y.T. Konttinen. 2007. MMPs, IL-1, and TNF are regulated by IL-17 in periodontitis. Journal of Dental Research 86: 347–351.
Li, W., L. Xiao, and J. Hu. 2013. Matrix metalloproteinase-1 promoter -1607 1G/2G polymorphism and chronic periodontitis susceptibility: a meta-analysis and systematic review. Journal of Clinical Periodontology 40(12): 1095–1103.
Kut-Lasserre, C., C.C. Miller, A.L. Ejeil, B. Gogly, M. Dridi, N. Piccardi, et al. 2001. Effect of avocado and soybean unsaponifiables ongelatinase A (MMP-2), stromelysin 1 (MMP-3), and tissue inhibitors of matrix metalloproteinase(TIMP- 1 and TIMP-2) secretion by human fibroblasts in culture. Journal of Periodontology 72: 1685–1694.
Cury, P.R., V.C. Araújo, F. Canavez, C. Furuse, and N.S. Araújo. 2007. Hydrocortisone affects the expression of matrix metalloproteinases (MMP-1, -2, -3, -7, and -11) and tissue inhibitor of matrix metalloproteinases (TIMP-1) in human gingival fibroblasts. Journal of Periodontology 78(7): 1309–1315.
Kubota, T., M. Itagaki, C. Hoshino, M. Nagata, T. Morozumi, T. Kobayashi, et al. 2008. Altered gene expression levels of matrix metalloproteinases and their inhibitors in periodontitis-affected gingival tissue. Journal of Periodontology 79: 166–173.
Tiranathanagul, S., T. Yongchaitrakul, K. Pattamapun, and P. Pavasant. 2004. Actinobacillus actinomycetemcomitans lipopolysaccharide activates matrix metalloproteinase-2 and increases receptor activator of nuclear factor-kappaB ligand expression in human periodontal ligament cells. Journal of Periodontology 75: 1647–1654.
Shimonishi, M., I. Takahashi, F. Terao, M. Komatsu, and M. Kikuchi. Induction of MMP-2 at the interface between epithelial cells and fibroblasts from human periodontal ligament. Journal of Periodontal Research 45:309-316.
Giannobile, W.V. 2008. Host-response therapeutics for periodontal diseases. Journal of Periodontology 79(8): 1592–600.
Yoo, T., S.A. Ham, J.S. Hwang, W.J. Lee, K.S. Paek, J.W. Oh, et al. 2016. Peroxisome proliferator-activated receptor δ inhibits Porphyromonas gingivalis lipopolysaccharide-induced activation of matrix metalloproteinase-2 by downregulating NADPH oxidase 4 in human gingival fibroblasts. Molecular Oral Microbiology 31(5): 398–409.
Kuo, P.J., H.P. Tu, Y.T. Chin, S.H. Lu, C.Y. Chiang, R.Y. Chen, et al. 2012. Cyclosporine-A inhibits MMP-2 and -9 activities in the presence of Porphyromonas gingivalis lipopolysaccharide: an experiment in human gingival fibroblast and U937 macrophage co-culture. Journal of Periodontology Research 47: 431–438.
Jenkins, K., M. Javadi, and R.C. Borghaei. 2004. Interleukin-4 suppresses IL-1-induced expression of matrix metalloproteinase-3 in human gingival fibroblasts. Journal of Periodontology 75: 283–291.
Verstappen, J., and J.W. Von den Hoff. 2006. Tissue inhibitors of metalloproteinases (TIMPs): their biological functions and involvement in oral disease. Journal of Dental Research 85(12): 1074–1084.
Ejeil, A.L., S. Igondjo-Tchen, S. Ghomrasseni, B. Pellat, G. Godeau, and B. Gogly. 2003. Expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in healthy and diseased human gingiva. Journal of Periodontology 74: 188–195.
Wang, P.L., and K. Ohura. 2002. Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts-CD14 and Toll-like receptors. Critical Reviews in Oral Biology and Medicine 13(2): 132–142.
Li, J.P., Y. Chen, C.H. Ng, M.L. Fung, A. Xu, B. Cheng, et al. 2014. Differential expression ofToll-like receptor 4 in healthy and diseased human gingiva. Journal of Periodontal Research 49(6): 845–854.
Scheres, N., M.L. Laine, P.M. Sipos, C.J. Bosch-Tijhof, W. Crielaard, T.J. de Vries, et al. 2011. Periodontal ligament and gingival fibroblasts from periodontitis patients are more active in interaction with Porphyromonas gingivalis. Journal of Periodontal Research 46(4): 407–416.
Andrukhov, O., S. Ertlschweiger, A. Moritz, H.P. Bantleon, and X. Rausch-Fan. 2014. Different effects of P.gingivalis LPS and E. coli LPS on the expression of interleukin-6 in human gingival fibroblasts. Acta Odontologica Scandinavica 72(5): 337–345.
Mahanonda, R., N. Sa-Ard-Iam, P. Montreekachon, A. Pimkhaokham, K. Yongvanichit, M.M. Fukuda, et al. 2007. IL-8 and IDO expression by human gingival fibroblasts via TLRs. Journal of Immunology 178: 1151–1157.
Baggiolini, M., B. Moser, and I. Clark-Lewis. 1994. Interleukin-8 and related chemotactic cytokines. Chest 105: 95–98.
Almasri, A., K. Wisithphrom, L.J. Windsor, and B. Olson. 2007. Nicotine and lipopolysaccharide affect cytokine expression from gingival fibroblasts. Journal of Periodontology 78: 533–541.
Kent, L.W., F. Rahemtulla, and S.M. Michalek. 1999. Interleukin (IL)-1 and Porphyromonas gingivalis lipopolysaccharide stimulation of IL-6 production by fibroblasts derived from healthy or periodontally diseased human gingival tissue. Journal of Periodontology 70: 274–282.
Takada, H., J. Mihara, I. Morisaki, and S. Hamada. 1991. Induction of interleukin-1 and -6 in human gingival fibroblast cultures stimulated with Bacteroides lipopolysaccharides. Infection and Immunity 59: 295–301.
Kubota, K., H. Sakaki, T. Imaizumi, H. Nakagawa, A. Kusumi, W. Kobayashi, et al. 2006. Retinoic acid-inducible gene-I is induced in gingival fibroblasts by lipopolysaccharide or poly IC: possible roles in interleukin-1beta, -6 and -8 expression. Oral Microbiology and Immunology 21: 399–406.
Wang, P.L., K. Sato, M. Oido, T. Fujii, Y. Kowashi, M. Shinohara, et al. 1998. Involvement of CD14 on human gingival fibroblasts in Porphyromonas gingivalis Lipopolysaccharide-mediated interleukin-6 secretion. Archives of Oral Biology 43: 687–694.
Imatani, T., T. Kato, and K. Okuda. 2001. Production of inflammatory cytokines by human gingival fibroblasts stimulated by cell-surface preparations of Porphyromonas gingivalis. Oral Microbiology and Immunology 16: 65–72.
Socransky, S.S., and A.D. Haffajee. 1992. The bacterial etiology of destructive periodontal disease: current concepts. Journal of Periodontology 63: 322–331.
Kocgozlu, L., R. Elkaim, H. Tenenbaum, and S. Werner. 2009. Variable cell responses to P. gingivalis lipopolysaccharide. Journal of Dental Research 88: 741–745.
Darveau, R.P., T.T. Pham, K. Lemley, R.A. Reife, B.W. Bainbridge, S.R. Coats, et al. 2004. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both toll-like receptors 2 and 4. Infection and Immunity 72: 5041–5051.
Tamura, M., M. Tokuda, S. Nagaoka, and H. Takada. 1992. Lipopolysaccharides of Bacteroides intermedius (Prevotella intermedia) and Bacteroides (Porphyromonas) gingivalis induce interleukin-8 gene expression in human gingival fibroblast cultures. Infection and Immunity 60: 4932–4937.
Irwin, C.R., T.T. Myrillas, P. Traynor, N. Leadbetter, and T.E. Cawston. 2002. The role of soluble interleukin (IL)-6 receptor in mediating the effects of IL-6 on matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 expression by gingival fibroblasts. Journal of Periodontology 73(7): 741–747.
Yanti, and J.K. Hwang. 2010. Suppressive effect of ethanolic Kaempferia pandurata Roxb. extract on matrix metalloproteinase-2 expression in Porphyromonas gingivalis-treated human gingival fibroblasts invitro. Journal of Oral Science 52: 583–591.
Zhang, W., F. Song, and L.J. Windsor. 2010. Effects of tobacco and P. gingivalis on gingival fibroblasts. Journal of Dental Research 89: 527–531.
Kato, T., N. Okahashi, S. Kawai, T. Kato, H. Inaba, I. Morisaki, et al. 2005. Impaired degradation of matrix collagen in human gingival fibroblasts by the antiepileptic drug phenytoin. Journal of Periodontology 76: 941–950.
Martelli-Junior, H., P. Cotrim, E. Graner, J.J. Sauk, and R.D. Coletta. 2003. Effect of transforming growth factor-beta1, interleukin-6, and interferon-gamma on the expression of type I collagen, heat shock protein47, matrix metalloproteinase (MMP)-1 and MMP-2 by fibroblasts from normal gingiva and hereditary gingival fibromatosis. Journal of Periodontology 74: 296–306.
Acknowledgments
This study was performed at the Selcuk University Faculty of Dentistry, Research Center and was supported by the Research Fund, Selcuk University (BAP/08201022).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bozkurt, S.B., Hakki, S.S., Hakki, E.E. et al. Porphyromonas gingivalis Lipopolysaccharide Induces a Pro-inflammatory Human Gingival Fibroblast Phenotype. Inflammation 40, 144–153 (2017). https://doi.org/10.1007/s10753-016-0463-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0463-7