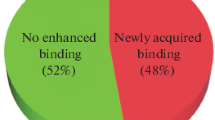
Abstract
Antibody repertoires of healthy humans and animals contain a fraction of antibodies able to acquire additional polyspecificity following exposure to several biologically relevant redox molecules (free heme, reactive oxygen species, ferrous ions, HOCl, etc.). The physiological role of these “hidden” polyspecific antibodies is poorly understood. Similar to inherently polyspecific antibodies, those with induced polyspecificicty may also have immunoregulatory properties. We have previously shown that a pooled human IgG preparation, modified by the exposure to ferrous ions, acquires the ability to significantly improve survival of animals with polymicrobial sepsis or aseptic systemic inflammation induced by bacterial lipopolysaccharide or zymosan administration. In the present study, we have analyzed the effects of administration of heme-exposed pooled human IgG in the same models of sepsis and aseptic systemic inflammation. The administration of a single dose of heme-exposed pooled IgG has resulted in a significant increase in the survival of mice with endotoxinemia, but not in those with polymicrobial sepsis and zymosan-induced severe generalized inflammation. Finally, we have provided evidence that the anti-inflammatory effect of heme-exposed IgG can be explained by scavenging of pro-inflammatory mediators.



Similar content being viewed by others
References
Notkins, A. 2004. Polyreactivity of antibody molecules. Trends in Immunology 25(4): 174–9.
Dimitrov, J.D., C. Planchais, L.T. Roumenina, T.L. Vassilev, S.V. Kaveri, and S. Lacroix-Desmazes. 2013. Antibody polyreactivity in health and disease: statu variabilis. Journal of Immunology 191(3): 993–9.
Zhou, Z.H., A.G. Tzioufas, and A.L. Notkins. 2007. Properties and function of polyreactive antibodies and polyreactive antigen-binding B cells. Journal of Autoimmunity 29(4): 219–228.
Zhou, Z.H., Y. Zhang, Y.F. Hu, L.M. Wahl, J.O. Cisar, and A.L. Notkins. 2007. The broad antibacterial activity of the natural antibody repertoire is due to polyreactive antibodies. Cell Host & Microbe 1(1): 51–61.
Dimitrov, J.D., N.D. Ivanovska, S. Lacroix-Desmazes, V.R. Doltchinkova, S.V. Kaveri, and T.L. Vassilev. 2006. Ferrous ions and reactive oxygen species increase antigen-binding and anti-inflammatory activities of immunoglobulin G. Journal of Biological Chemistry 281(1): 439–446.
Dimitrov, J.D., C. Planchais, J. Kang, A. Pashov, T.L. Vassilev, S.V. Kaveri, and S. Lacroix-Desmazes. 2010. Heterogeneous antigen recognition behavior of induced polyspecific antibodies. Biochemical and Biophysical Research Communications 398(2): 266–271.
Dimitrov, J.D., L.T. Roumenina, V.R. Doltchinkova, N.M. Mihaylova, S. Lacroix-Desmazes, S.V. Kaveri, and T.L. Vassilev. 2007. Antibodies use heme as a cofactor to extend their pathogen elimination activity and to acquire new effector functions. Journal of Biological Chemistry 282(37): 26696–26706.
Dimitrov, J.D., T.L. Vassilev, S. Andre, S.V. Kaveri, and S. Lacroix-Desmazes. 2008. Functional variability of antibodies upon oxidative processes. Autoimmunity Reviews 7(7): 574–578.
McIntyre, J.A., D.R. Wagenknecht, and W.P. Faulk. 2005. Autoantibodies unmasked by redox reactions. Journal of Autoimmunity 24(4): 311–317.
Mihaylova, N.M., J.D. Dimitrov, I.K. Djoumerska-Alexieva, and T.L. Vassilev. 2008. Inflammation-induced enhancement of IgG immunoreactivity. Inflammation Research 57(1): 1–3.
Kazatchkine, M.D., and S.V. Kaveri. 2001. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. New England Journal of Medicine 345(10): 747–755.
Djoumerska-Alexieva, I., L. Roumenina, A. Pashov, J. Dimitrov, M. Hadzhieva, S. Lindig, et al. 2015. Intravenous immunoglobulin with enhanced polyspecificity improves survival in experimental sepsis and aseptic systemic inflammatory response syndromes. Molecular Medicine 21: 1002–1010.
Pavlovic, S., N. Zdravkovic, J.D. Dimitrov, A. Djukic, N. Arsenijevic, T.L. Vassilev, and M.L. Lukic. 2011. Intravenous immunoglobulins exposed to heme (heme IVIG) are more efficient than IVIG in attenuating autoimmune diabetes. Clinical Immunology 138(2): 162–171.
Xiao, W., M.N. Mindrinos, J. Seok, J. Cuschieri, A.G. Cuenca, H. Gao, et al. 2011. A genomic storm in critically injured humans. Journal of Experimental Medicine 208(13): 2581–2590.
Roumenina, L.T., J. Rayes, S. Lacroix-Desmazes, and J.D. Dimitrov. 2016. Heme: modulator of plasma systems in hemolytic diseases. Trends in Molecular Medicine 22(3): 200–213.
Lecerf, M., T. Scheel, A.D. Pashov, A. Jarossay, D. Ohayon, C. Planchais, et al. 2015. Prevalence and gene characteristics of antibodies with cofactor-induced HIV-1 specificity. Journal of Biological Chemistry 290(8): 5203–5213.
Larsen, R., R. Gozzelino, V. Jeney, L. Tokaji, F.A. Bozza, A.M. Japiassu, et al. 2010. A central role for free heme in the pathogenesis of severe sepsis. Science Translational Medicine 2(51): 51ra71.
Brocklehurst, P., B. Farrell, A. King, E. Juszczak, B. Darlow, K. Haque, A. Salt, B. Stenson, and W. Tarnow-Mordi. 2011. Treatment of neonatal sepsis with intravenous immune globulin. New England Journal of Medicine 365(13): 1201–1211.
Dimitrov, J.D., C. Planchais, T. Scheel, D. Ohayon, S. Mesnage, C. Berek, S.V. Kaveri, and S. Lacroix-Desmazes. 2014. A cryptic polyreactive antibody recognizes distinct clades of HIV-1 glycoprotein 120 by an identical binding mechanism. Journal of Biological Chemistry 289(25): 17767–17779.
Wang, H., O. Bloom, M. Zhang, J.M. Vishnubhakat, M. Ombrellino, J. Che, et al. 1999. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285(5425): 248–251.
Xu, J., X. Zhang, R. Pelayo, M. Monestier, C.T. Ammollo, F. Semeraro, et al. 2009. Extracellular histones are major mediators of death in sepsis. Nature Medicine 15(11): 1318–1321.
Qiang, X., W.L. Yang, R. Wu, M. Zhou, A. Jacob, W. Dong, et al. 2013. Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nature Medicine 19(11): 1489–1495.
Czermak, B.J., V. Sarma, C.L. Pierson, R.L. Warner, M. Huber-Lang, N.M. Bless, H. Schmal, H.P. Friedl, and P.A. Ward. 1999. Protective effects of C5a blockade in sepsis. Nature Medicine 5(7): 788–792.
Riedemann, N.C., R.F. Guo, and P.A. Ward. 2003. Novel strategies for the treatment of sepsis. Nature Medicine 9(5): 517–524.
Rittirsch, D., M.A. Flierl, B.A. Nadeau, D.E. Day, M. Huber-Lang, C.R. Mackay, et al. 2008. Functional roles for C5a receptors in sepsis. Nature Medicine 14(5): 551–557.
Volman, T.J., T. Hendriks, and R.J. Goris. 2005. Zymosan-induced generalized inflammation: experimental studies into mechanisms leading to multiple organ dysfunction syndrome. Shock 23(4): 291–297.
Hubbard, W.J., M. Choudhry, M.G. Schwacha, J.D. Kerby, L.W. Rue 3rd, K.I. Bland, and I.H. Chaudry. 2005. Cecal ligation and puncture. Shock 24(Suppl 1): 52–57.
Acknowledgments
This work was supported by grants from the Bulgarian Science Fund (grant DFNI B02/29) and from the Agence Nationale de la Recherche (ANR-13-JCV1-006-01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experimental protocols were approved by the Animal Care Commission of the Institute of Microbiology in accordance with National and European Regulations (BABH protocol #105/10 July 2014).
ELECTRONIC SUPPLEMENTARY MATERIAL
Below is the link to the electronic supplementary material.
ESM 1
(JPG 32 kb)
Rights and permissions
About this article
Cite this article
Djoumerska-Alexieva, I., Roumenina, L.T., Stefanova, T. et al. Heme-Exposed Pooled Therapeutic IgG Improves Endotoxemia Survival. Inflammation 40, 117–122 (2017). https://doi.org/10.1007/s10753-016-0460-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0460-x