Abstract
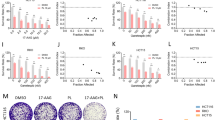
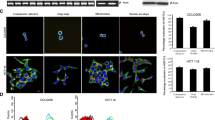
The existence of multiple-interactive roles between several signaling pathways in tumorigenesis shows the significance of pharmacological factors like heat shock protein 90 (HSP90) inhibitors which control several signaling pathways simultaneously. HSP90 as a molecular chaperone supports the active conformational structure and function of several oncogenic signal proteins, termed “client” proteins, some of them act as a link between cancer and inflammation. Prostaglandin E2 (PGE2) is one of the major mediators of inflammation in colorectal cancer development and progress. However, the relationship between chaperone activity of HSP90 and PGE2 levels remains unclear. We evaluated the inhibitory effects of 17-demethoxy-17-allylamino geldanamycin (1 7-AAG), an HSP90 inhibitor, on PGE2 levels in HT-29 colorectal cancer cells. For the first time, we showed inhibitory effects of 17-AAG, on PGE2 levels in HT-29 colorectal cancer cells. 17-AAG inhibited PMA-induced cyclooxygenase-2 (COX-2) mRNA expression and protein level. We showed 15-hydroxyprostaglandin dehydrogenase (15-PGDH) expression induced by 17-AAG treatment at both mRNA and protein levels. In conclusion, we found that inhibitory effects of 17-AAG on PGE2 levels in HT-29 colorectal cancer cells were mediated through modulating COX-2 and 15-PGDH expression.




Similar content being viewed by others
References
Ricchi, P., R. Zarrilli, A. Di Palma, and A.M. Acquaviva. 2003. Nonsteroidal anti-inflammatory drugs in colorectal cancer: from prevention to therapy. British Journal of Cancer 88: 803–7.
Tuynman, J.B., M.P. Peppelenbosch, and D.J. Richel. 2004. COX-2 inhibition as a tool to treat and prevent colorectal cancer. Critical Reviews in Oncology/Hematology 52: 81–101.
Goetz, M.P., D.O. Toft, M.M. Ames, and C. Erlichman. 2003. The HSP90 chaperone complex as a novel target for cancer therapy. Annals of Oncology 14: 1169–76.
Khanna, M., F. Wang, I. Jo, W.E. Knabe, S.M. Wilson, L. Li, K. Bum-Erdene, J. Li, G. W Sledge, R. Khanna, and S.O. Meroueh. 2011. Targeting multiple conformations leads to small molecule inhibitors of the uPAR.uPA protein-protein interaction that block cancer cell invasion. ACS Chemical Biology 6: 1232–43.
Li, Y., T. Zhang, S.J. Schwartz, and D. Sun. 2009. New developments in HSP90 inhibitors as anti-cancer therapeutics: mechanisms, clinical perspective and more potential. Drug Resistance Updates 12: 17–27.
Bai, L., S. Xu, W. Chen, Z. Li, X. Wang, H. Tang, and Y. Ling. 2011. Blocking NF-kappaB and Akt by HSP90 inhibition sensitizes Smac mimetic compound 3-induced extrinsic apoptosis pathway and results in synergistic cancer cell death. Apoptosis 16: 45–54.
Wang, X., W. Ju, J. Renouard, J. Aden, S.A. Belinsky, and Y. Lin. 2006. 17-allylamino-17-demethoxygeldanamycin synergistically potentiates tumor necrosis factor-induced lung cancer cell death by blocking the nuclear factor-kappaB pathway. Cancer Research 66: 1089–95.
Lilja, A., C.E. Weeden, K. McArthur, T. Nguyen, A. Donald, Z.X. Wong, L. Dousha, S. Bozinovski, R. Vlahos, C.J. Burns, M.L. Asselin-Labat, et al. 2015. HSP90 inhibition suppresses lipopolysaccharide-induced lung inflammation in vivo. PLoS One 10, e0114975.
Sevin M, Girodon F, Garrido C, and de Thonel A. HSP90 and HSP70: Implication in inflammation processes and therapeutic approaches for myeloproliferative neoplasms. 2015. Mediators Inflamm: 970242. doi: 10.1155/2015/970242.
Hance, M.W., K.D. Nolan, and J.S. Isaacs. 2014. The double-edged sword: conserved functions of extracellular HSP90 in wound healing and cancer. Cancers (Basel) 6: 1065–97.
Chen, W., Z. Li, L. Bai, and Y. Lin. 2011. NF-kappa B in lung cancer, a carcinogenesis mediator and a prevention and therapy target. Frontiers in Bioscience (Landmark Edition) 16: 1172–85.
Wang, D., and R.N. DuBois. 2014. PPAR delta and PGE signaling pathways communicate and connect inflammation to colorectal cancer. Inflamm Cell Signal 1, 10.14800/ics.338.
Na, H.K., J.M. Park, H.G. Lee, H.N. Lee, S.J. Myung, and Y.J. Surh. 2011. 15-Hydroxyprostaglandin dehydrogenase as a novel molecular target for cancer chemoprevention and therapy. Biochemical Pharmacology 82: 1352–60.
Nakanishi, M., and D.W. Rosenberg. 2013. Multifaceted roles of PGE2 in inflammation and cancer. Seminars in Immunopathology 35: 123–37.
Castro-Sanchez, L., N. Agra, C. LlorenteIzquierdo, O. Motino, M. Casado, L. Bosca, and P. Martín-Sanz. 2013. Regulation of 15-hydroxyprostaglandin dehydrogenase expression in hepatocellular carcinoma. The International Journal of Biochemistry & Cell Biology 45: 2501–11.
Ataee, R., S. Ajdary, M. Rezayat, M.A. Shokrgozar, S. Shahriari, and M.R. Zarrindast. 2010. Study of 5HT3 and HT4 receptor expression in HT-29 cell line and human colon adenocarcinoma tissues. Archives of Iranian Medicine 13: 120–5.
Ruijter, J.M., C. Ramakers, W.M. Hoogaars, Y. Karlen, O. Bakker, M.J. van den Hoff, and A.F. Moorman. 2009. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Research 37, e45.
Xian, Y.F., Y.C. Li, S.P. Ip, Z.X. Lin, X.P. Lai, and Z.R. Su. 2011. Anti-inflammatory effect of patchouli alcohol isolated from Pogostemonis Herba in LPS-stimulated RAW264.7 macrophages. ExpTher Med 2: 545–50.
Choi, Y.E., C. Battelli, J. Watson, J. Liu, J. Curtis, A.N. Morse, U.A. Matulonis, D. Chowdhury, and P.A. Konstantinopoulos. 2014. Sublethal concentrations of 17-AAG suppress homologous recombination DNA repair and enhance sensitivity to carboplatin and olaparib in HR proficient ovarian cancer cells. Oncotarget 5: 2678–87.
Kacimi, R., and M.A. Yenari. 2015. Pharmacologic heat shock protein 70 induction confers cytoprotection against inflammation in gliovascular cells. Glia 63: 1200–12.
Kaltschmidt, B., R.A. Linker, J. Deng, and C. Kaltschmidt. 2002. Cyclooxygenase-2 is a neuronal target gene of NF-kappaB. BMC Molecular Biology 3: 16.
Zarghi, A., and S. Arfaei. 2011. Selective COX-2 inhibitors: a review of their structure-activity relationships. Iran Journal of Pharmacy Research 10: 655–83.
Holla, V.R., J.R. Mann, Q. Shi, and R.N. DuBois. 2006. Prostaglandin E2 regulates the nuclear receptor NR4A2 in colorectal cancer. The Journal of Biological Chemistry 281: 2676–82.
Haggar, F.A., and R.P. Boushey. 2009. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clinical Colon & Rectal Surgery 22: 191–7.
Weinstein, I.B., and A.K. Joe. 2006. Mechanisms of disease: oncogene addiction—a rationale for molecular targeting in cancer therapy. Nature Clinical Practice. Oncology 3: 448–57.
Kang, S., A. Min, S.A. Im, S.H. Song, S.G. Kim, H.A. Kim, H.J. Kim, D.Y. Oh, H.S. Jong, T.Y. Kim, and Y.J. Bang. 2015. TGF-beta suppresses COX-2 expression by tristetraprolin-mediated RNA destabilization in A549 human lung cancer cells. Cancer Research and Treatment 47: 101–9.
Lee, K.H., A.H. Jang, and C.G. Yoo. 2015. 17-Allylamino-17-demethoxygeldanamycin and the enhancement of PS-341-induced lung cancer cell death by blocking the NF-kappaB and PI3K/Akt pathways. American Journal of Respiratory Cell and Molecular Biology 53: 412–21.
Reti, A., G. Barna, E. Pap, V. Adleff, V. LK, A. Jeney, J. Kralovánszky, and B. Budai. 2009. Enhancement of 5-fluorouracil efficacy on high COX-2 expressing HCA-7 cells by low dose indomethacin and NS-398 but not on low COX-2 expressing HT-29 cells. Pathology Oncology Research 15: 335–44.
Kaidi, A., D. Qualtrough, A.C. Williams, and C. Paraskeva. 2006. Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Research 66: 6683–91.
Csiki, I., K. Yanagisawa, N. Haruki, S. Nadaf, J.D. Morrow, D.H. Johnson, and D.P. Carbone. 2006. Thioredoxin-1 modulates transcription of cyclooxygenase-2 via hypoxia-inducible factor-1 alpha in non-small cell lung cancer. Cancer Research 66: 143–50.
Lu, D., C. Han, and T. Wu. 2014. 15-PGDH inhibits hepatocellular carcinoma growth through 15-keto-PGE2/PPAR gamma-mediated activation of p21WAF1/Cip1. Oncogene 33: 1101–12.
Chi, X., B.M. Freeman, M. Tong, Y. Zhao, and H.H. Tai. 2009. 15-Hydroxyprostaglandin dehydrogenase (15-PGDH) is up-regulated by flurbiprofen and other non-steroidal anti-inflammatory drugs in human colon cancer HT29 cells. Archives of Biochemistry and Biophysics 487: 139–45.
Wakimoto, N., I. Wolf, D. Yin, J. O’Kelly, T. Akagi, L. Abramovitz, K.L. Black, H.H. Tai, and H.P. Koeffler. 2008. Nonsteroidal anti-inflammatory drugs suppress glioma via 15-hydroxyprostaglandin dehydrogenase. Cancer Research 68: 6978–86.
Casciani, V., E. Marinoni, A.D. Bocking, M. Moscarini, R. Di Iorio, and J.R. Challis. 2008. Opposite effect of phorbol ester PMA on PTGS2 and PGDH mRNA expression in human choriontrophoblast cells. Reproductive Sciences 15: 40–50.
Tong, M., Y. Ding, and H.H. Tai. 2006. Reciprocal regulation of cyclooxygenase-2 and 15-hydroxyprostaglandin dehydrogenase expression in A549 human lung adenocarcinoma cells. Carcinogenesis 27: 2170–9.
Jang, T.J., K.H. Jeon, and K.H. Jung. 2009. Cyclooxygenase-2 expression is related to the epithelial-to-mesenchymal transition in human colon cancers. Yonsei Medical Journal 31: 818–24.
Chandramouli, A., M.E. Mercado-Pimentel, A. Hutchinson, A. Gibadulinova, E.R. Olson, S. Dickinson, R. Shañas, J. Davenport, J. Owens, A.K. Bhattacharyya, J.W. Regan, et al. 2010. The induction of S100p expression by the prostaglandin E(2) (PGE(2))/EP4 receptor signaling pathway in colon cancer cells. Cancer Biology & Therapy 10: 1056–66.
Dufour, M., S. Faes, A. Dormond-Meuwly, N. Demartines, and O. Dormond. 2014. PGE2-induced colon cancer growth is mediated by mTORC1. Biochemical and Biophysical Research Communications 451: 587–91.
Altorki, N.K., K. Subbaramaiah, and A.J. Dannenberg. 2003. Cyclooxygenase-2: a target for the prevention and treatment of cancers of the upper digestive tract. Progress in Experimental Tumor Research 37: 107–23.
Solomon, S.D., J.J. McMurray, M.A. Pfeffer, J. Wittes, R. Fowler, P. Finn, W.F. Anderson, A. Zauber, E. Hawk, and M. Bertagnolli. 2005. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. New England Journal of Medicine 352: 1071–80.
Acknowledgments
The authors thank the Deputy of Research Affairs of Kerman University of Medical Sciences for funding this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mohammadi, A., Yaghoobi, M., Gholamhoseinian Najar, A. et al. HSP90 Inhibition Suppresses PGE2 Production via Modulating COX-2 and 15-PGDH Expression in HT-29 Colorectal Cancer Cells. Inflammation 39, 1116–1123 (2016). https://doi.org/10.1007/s10753-016-0343-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0343-1