Abstract
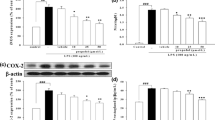
High-mobility group box 1 (HMGB1) plays a key role in the development of acute lung injury (ALI). Propofol, a general anesthetic with anti-inflammatory properties, has been suggested to be able to modulate lipopolysaccharide (LPS)-induced ALI. In this study, we investigated the effects of propofol on the expression of HMGB1 in a rat model of LPS-induced ALI. Rats underwent intraperitoneal injection of LPS to mimic sepsis-induced ALI. Propofol bolus (1, 5, or 10 mg/kg) was infused continuously 30 min after LPS administration, followed by infusion at 5 mg/(kg · h) through the left femoral vein cannula. LPS increased wet to dry weight ratio and myeloperoxidase activity in lung tissues and caused the elevation of total protein and cells, neutrophils, macrophages, and neutrophils in bronchoalveolar lavage fluid (BALF). Moreover, HMGB1 and other cytokine levels were increased in BALF and lung tissues and pathological changes of lung tissues were excessively aggravated in rats after LPS administration. Propofol inhibited all the above effects. It also inhibited LPS-induced toll-like receptor (TLR)2/4 protein upexpression and NF-κB activation in lung tissues and human alveolar epithelial cells. Propofol protects rats and human alveolar epithelial cells against HMGB1 expression in a rat model of LPS-induced ALI. These effects may partially result from reductions in TLR2/4 and NF-κB activation.









Similar content being viewed by others
References
Su, C.F., S.J. Kao, and H.I. Chen. 2012. Acute respiratory distress syndrome and lung injury: pathogenetic mechanism and therapeutic implication. World Journal Critical Care Medicine 1: 50–60.
Castellheim, A., O.L. Brekke, T. Espevik, M. Harboe, and T.E. Mollnes. 2009. Innate immune responses to danger signals in systemic inflammatory response syndrome and sepsis. Scandinavian Journal of Immunology 69: 479–491.
Jing, H., J. Yao, X. Liu, H. Fan, F. Zhang, Z. Li, X. Tian, and Y. Zhou. 2014. Fish-oil emulsion (omega-3 polyunsaturated fatty acids) attenuates acute lung injury induced by intestinal ischemia-reperfusion through adenosine 5′-monophosphate-activated protein kinase-sirtuin1 pathway. Journal of Surgical Research 187: 252–261.
Song, Z., X. Zhao, Y. Gao, M. Liu, M. Hou, H. Jin, and Y. Cui. 2015. Recombinant human brain natriuretic peptide ameliorates trauma-induced acute lung injury via inhibiting JAK/STAT signaling pathway in rats. Journal Trauma Acute Care Surgery 78: 980–987.
Lin, W.C., C.W. Chen, Y.W. Huang, L. Chao, J. Chao, Y.S. Lin, and C.F. Lin. 2015. Kallistatin protects against sepsis-related acute lung injury via inhibiting inflammation and apoptosis. Scientific Reports 5: 12463.
Lu, B., C. Wang, M. Wang, W. Li, F. Chen, K.J. Tracey, and H. Wang. 2014. Molecular mechanism and therapeutic modulation of high mobility group box 1 release and action: an updated review. Expert Review of Clinical Immunology 10: 713–727.
Agalave, N.M., and C.I. Svensson. 2015. Extracellular high-mobility group box 1 protein (HMGB1) as a mediator of persistent pain. Molecular Medicine 20: 569–578.
Wang, H., M.F. Ward, and A.E. Sama. 2014. Targeting HMGB1 in the treatment of sepsis. Expert Opinion on Therapeutic Targets 18: 257–268.
Wang, H., O. Bloom, M. Zhang, J.M. Vishnubhakat, M. Ombrellino, J. Che, A. Frazier, H. Yang, S. Ivanova, L. Borovikova, K.R. Manogue, E. Faist, E. Abraham, J. Andersson, U. Andersson, P.E. Molina, N.N. Abumrad, A. Sama, and K.J. Tracey. 1999. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285: 248–251.
Lutz, W., and J. Stetkiewicz. 2004. High mobility group box 1 protein as a late-acting mediator of acute lung inflammation. International Journal of Occupational Medicine and Environmental Health 17: 245–254.
Yang, H., M. Ochani, J. Li, X. Qiang, M. Tanovic, H.E. Harris, S.M. Susarla, L. Ulloa, H. Wang, R. DiRaimo, C.J. Czura, J. Roth, H.S. Warren, M.P. Fink, M.J. Fenton, U. Andersson, and K.J. Tracey. 2004. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proceedings of the National Academy of Sciences of the United States of America 101: 296–301.
Ye, C., J.G. Choi, S. Abraham, H. Wu, D. Diaz, D. Terreros, P. Shankar, and N. Manjunath. 2012. Human macrophage and dendritic cell-specific silencing of high-mobility group protein B1 ameliorates sepsis in a humanized mouse model. Proceedings of the National Academy of Sciences of the United States of America 109: 21052–21057.
van Beijnum, J.R., W.A. Buurman, and A.W. Griffioen. 2008. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis 11: 91–99.
Park, J.S., F. Gamboni-Robertson, Q. He, D. Svetkauskaite, J.Y. Kim, D. Strassheim, J.W. Sohn, S. Yamada, I. Maruyama, A. Banerjee, A. Ishizaka, and E. Abraham. 2006. High mobility group box 1 protein interacts with multiple toll-like receptors. American Journal of Physiology - Cell Physiology 290: C917–C924.
Kim, S., S.Y. Kim, J.P. Pribis, M. Lotze, K.P. Mollen, R. Shapiro, P. Loughran, M.J. Scott, and T.R. Billiar. 2013. Signaling of high mobility group box 1 (HMGB1) through toll-like receptor 4 in macrophages requires CD14. Molecular Medicine 19: 88–98.
Hirata, Y., H. Kurobe, M. Higashida, D. Fukuda, M. Shimabukuro, K. Tanaka, Y. Higashikuni, T. Kitagawa, and M. Sata. 2013. HMGB1 plays a critical role in vascular inflammation and lesion formation via toll-like receptor 9. Atherosclerosis 231: 227–233.
Bao, H.G., and S. Li. 2011. Effects of propofol on the outcomes of rats with sepsis. Journal of Surgical Research 168: e111–e115.
Song, X.M., Y.L. Wang, J.G. Li, C.Y. Wang, Q. Zhou, Z.Z. Zhang, and H. Liang. 2009. Effects of propofol on pro-inflammatory cytokines and nuclear factor kappaB during polymicrobial sepsis in rats. Molecular Biology Reports 36: 2345–2351.
Chiu, W.T., Y.L. Lin, C.W. Chou, and R.M. Chen. 2009. Propofol inhibits lipoteichoic acid-induced iNOS gene expression in macrophages possibly through downregulation of toll-like receptor 2-mediated activation of Raf-MEK1/2-ERK1/2-IKK-NFkappaB. Chemico-Biological Interactions 181: 430–439.
Ma, L., X.Y. Wu, L.H. Zhang, W.M. Chen, A. Uchiyama, T. Mashimo, and Y. Fujino. 2013. Propofol exerts anti-inflammatory effects in rats with lipopolysaccharide-induced acute lung injury by inhibition of CD14 and TLR4 expression. Brazilian Journal of Medical and Biological Research 46: 299–305.
Wang, T., X.Y. Wei, B. Liu, L.J. Wang, and L.H. Jiang. 2015. Effects of propofol on lipopolysaccharide-induced expression and release of HMGB1 in macrophages. Brazilian Journal of Medical and Biological Research 48: 286–291.
Liang, C., J. Cang, H. Wang, and Z. Xue. 2013. Propofol attenuates cerebral ischemia/reperfusion injury partially using heme oxygenase-1. Journal of Neurosurgical Anesthesiology 25: 311–316.
Shen, W., J. Gan, S. Xu, G. Jiang, and H. Wu. 2009. Penehyclidine hydrochloride attenuates LPS-induced acute lung injury involvement of NF-kappaB pathway. Pharmacological Research 60: 296–302.
Chu, C.H., D. David Liu, Y.H. Hsu, K.C. Lee, and H.I. Chen. 2007. Propofol exerts protective effects on the acute lung injury induced by endotoxin in rats. Pulmonary Pharmacology & Therapeutics 20: 503–512.
Li, S., H. Bao, L. Han, and L. Liu. 2010. Effects of propofol on early and late cytokines in lipopolysaccharide-induced septic shock in rats. Journal Biomedical Research 24: 389–394.
Wei, L., H. Matsumoto, and H. Yamaguchi. 2013. Propofol attenuates lipopolysaccharide-induced monocyte chemoattractant protein-1 production through p38 MAPK and SAPK/JNK in alveolar epithelial cells. Journal of Anesthesia 27: 366–373.
Zhao L.L., Hu G.C., Zhu S.S., Li J.F. and Liu G.J. 2014. Propofol pretreatment attenuates lipopolysaccharide-induced acute lung injury in rats by activating the phosphoinositide-3-kinase/Akt pathway. Brazilian Journal of Medical and Biological Research.
Gokcinar, D., V. Ergin, A. Cumaoglu, A. Menevse, and A. Aricioglu. 2013. Effects of ketamine, propofol, and ketofol on proinflammatory cytokines and markers of oxidative stress in a rat model of endotoxemia-induced acute lung injury. Acta Biochimica Polonica 60: 451–456.
Feng, G., B. Sun, and T.Z. Li. 2015. Daidzein attenuates lipopolysaccharide-induced acute lung injury via toll-like receptor 4/NF-kappaB pathway. International Immunopharmacology 26: 392–400.
Tianzhu, Z., and W. Shumin. 2015. Esculin inhibits the inflammation of LPS-induced acute lung injury in mice via regulation of TLR/NF-kappaB pathways. Inflammation 38: 1529–1536.
Kawasaki, T., and T. Kawai. 2014. Toll-like receptor signaling pathways. Frontiers in Immunology 5: 461.
Chang, Y., X. Huang, Z. Liu, G. Han, L. Huang, Y.C. Xiong, and Z. Wang. 2013. Dexmedetomidine inhibits the secretion of high mobility group box 1 from lipopolysaccharide-activated macrophages in vitro. Journal of Surgical Research 181: 308–314.
Liu, Z., J. Zhang, X. Huang, L. Huang, S. Li, and Z. Wang. 2013. Magnesium sulfate inhibits the secretion of high mobility group box 1 from lipopolysaccharide-activated RAW264.7 macrophages in vitro. Journal of Surgical Research 179: e189–e195.
Liu, Z., Y. Chang, J. Zhang, X. Huang, J. Jiang, S. Li, and Z. Wang. 2013. Magnesium deficiency promotes secretion of high-mobility group box 1 protein from lipopolysaccharide-activated macrophages in vitro. Journal of Surgical Research 180: 310–316.
Yang, Q., X. Liu, Z. Yao, S. Mao, Q. Wei, and Y. Chang. 2014. Penehyclidine hydrochloride inhibits the release of high-mobility group box 1 in lipopolysaccharide-activated RAW264.7 cells and cecal ligation and puncture-induced septic mice. Journal of Surgical Research 186: 310–317.
Mackenzie, N., and I.S. Grant. 1987. Propofol for intravenous sedation. Anaesthesia 42: 3–6.
Shao, H., J. Li, Y. Zhou, Z. Ge, J. Fan, Z. Shao, and Y. Zeng. 2008. Dose-dependent protective effect of propofol against mitochondrial dysfunction in ischaemic/reperfused rat heart: role of cardiolipin. British Journal of Pharmacology 153: 1641–1649.
Li, J., B. Han, X. Ma, and S. Qi. 2010. The effects of propofol on hippocampal caspase-3 and Bcl-2 expression following forebrain ischemia-reperfusion in rats. Brain Research 1356: 11–23.
Karashima, Y., M. Oike, S. Takahashi, and Y. Ito. 2002. Propofol prevents endothelial dysfunction induced by glucose overload. British Journal of Pharmacology 137: 683–691.
Wang, B., T. Luo, D. Chen, and D.M. Ansley. 2007. Propofol reduces apoptosis and up-regulates endothelial nitric oxide synthase protein expression in hydrogen peroxide-stimulated human umbilical vein endothelial cells. Anesthesia and Analgesia 105: 1027–1033. Table of contents.
Vasile, B., F. Rasulo, A. Candiani, and N. Latronico. 2003. The pathophysiology of propofol infusion syndrome: a simple name for a complex syndrome. Intensive Care Medicine 29: 1417–1425.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSF 30872433).
Author Contributions
Xiaoyan Wang: conception, design, analysis, and interpretation of data; writing the manuscript.
Gongming Wang: conception, design, analysis, and interpretation of data; writing the manuscript.
Chengxiao Liu: analysis and interpretation of data; writing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures were performed in accordance with the Declaration of Helsinki of the World Medical Association. The study was approved by the ethics committee of Shandong Provincial Hospital Affiliated to Shandong University, Jinan, Shandong, China.
Conflicts of Interest
The authors report no proprietary or commercial interest in any product mentioned, or concept discussed, in this article.
Rights and permissions
About this article
Cite this article
Wang, X., Liu, C. & Wang, G. Propofol Protects Rats and Human Alveolar Epithelial Cells Against Lipopolysaccharide-Induced Acute Lung Injury via Inhibiting HMGB1 Expression. Inflammation 39, 1004–1016 (2016). https://doi.org/10.1007/s10753-016-0330-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0330-6