Abstract
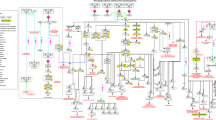
To achieve the goal of identifying the gene groups that regulated macrophage activation, a total of 925 differentially expressed genes of activated macrophages were found at the intersection of the three series (GSE5099-1, GSE5099-2, and GSE18686) from the Gene Expression Omnibus (GEO) database, and a sub-network was constructed based on the protein-protein interaction (PPI) network. Four communities (K = 3) were identified from the sub-network using the CFinder software. Community 1 was considered as the gene group of interest base on the heat map. GO-BP and KEGG enrichment analysis with the DAVID software showed that the functions of the 14 genes in community 1 were mainly related to the NF-κB pathway. A network was constructed using the Cytoscape software. The diagram showed that STAT1, NFKBIA, NFKAIB, JUN, and RELA were the key genes in the regulation of macrophage activation. Among these genes, RELA (NF-κB P65) was an important member of the NF-κB family, while NFKBIA (IκBα) and NFKAIB (IκBβ) were the inhibitory factors of NF-κB. Small molecules capable of regulating these five genes were identified via the CMap software, and a network diagram was generated using the Cytoscape software to provide a reference for the development of new drugs that regulate macrophage activation.








Similar content being viewed by others
REFERENCES
Shifrin, A.L., N. Chirmule, Y. Zhang, and S.E. Raper. 2005. Macrophage ablation attenuates adenoviral vector-induced pancreatitis. Surgery 137(5): 545–551. doi:10.1016/j.surg.2005.01.004. PMID: 15855927.
Shah, A.U., A. Sarwar, A.I. Orabi, S. Gautam, W.M. Grant, A.J. Park, A.U. Shah, et al. 2009. Protease activation during in vivo pancreatitis is dependent on calcineurin activation. American Journal of Physiology-Gastrointestinal and Liver Physiology 297(5): G967–G973. doi:10.1152/ajpgi.00181.2009. PMID: 20501444.
Mayerle, J., A. Dummer, M. Sendler, S.R. Malla, C. van den Brandt, S. Teller, A. Aghdassi, C. Nitsche, and M.M. Lerch. 2012. Differential roles of inflammatory cells in pancreatitis. Journal of Gastroenterology and Hepatology 27(Suppl 2): 47–51. doi:10.1111/j.1440-1746.2011.07011.x. PMID: 22320916.
Franco-Pons, N., S. Gea-Sorli, and D. Closa. 2010. Release of inflammatory mediators by adipose tissue during acute pancreatitis. Journal of Pathology 221(2): 175–182. doi:10.1002/path.2691. PMID: 20217859.
Cao, J., and Q. Liu. 2013. Protective effects of sivelestat in a caerulein-induced rat acute pancreatitis model. Inflammation 36(6): 1348–1356. doi:10.1007/s10753-013-9674-3. PMID: 23794035.
Takei, M., M. Kobayashi, D.N. Herndon, R.B. Pollard, and F. Suzuki. 2006. Glycyrrhizin inhibits the manifestations of anti-inflammatory responses that appear in association with systemic inflammatory response syndrome (SIRS)-like reactions. Cytokine 35(5–6): 295–301. doi:10.1016/j.cyto.2006.10.002. PMID: 17113306.
Martinez, F.O., S. Gordon, M. Locati, and A. Mantovani. 2006. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. Journal of Immunology 177(10): 7303–7311. PMID: 17082649.
Solinas, G., S. Schiarea, M. Liguori, M. Fabbri, S. Pesce, L. Zammataro, F. Pasqualini, et al. 2010. Tumor-conditioned macrophages secrete migration-stimulating factor: A new marker for M2-polarization, influencing tumor cell motility. Journal of Immunology 185(1): 642–652. doi:10.4049/jimmunol.1000413. PMID: 20530259.
Fuentes-Duculan, J., M. Suarez-Farinas, L.C. Zaba, K.E. Nograles, K.C. Pierson, H. Mitsui, C.A. Pensabene, et al. 2010. A subpopulation of CD163-positive macrophages is classically activated in psoriasis. Journal of Investigative Dermatology 130(10): 2412–2422. doi:10.1038/jid.2010.165. PMID: 20555352.
Bolstad, B.M., R.A. Irizarry, M. Astrand, and T.P. Speed. 2003. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19(2): 185–193. doi:10.1093/bioinformatics /19.2.185. PMID:12538238.
Irizarry, R.A., B.M. Bolstad, F. Collin, L.M. Cope, B. Hobbs, and T.P. Speed. 2003. Summaries of affymetrix GeneChip probe level data. Nucleic Acids Research 31(4): e15. doi:10.1093/nar/gng015. PMID:12582260.
Irizarry, R.A., B. Hobbs, F. Collin, Y.D. Beazer-Barclay, K.J. Antonellis, U. Scherf, and T.P. Speed. 2003. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4(2): 249–264. doi:10.1093/ biostatistics/4.2.249. PMID:12925520.
Jonsson, P.F., and P.A. Bates. 2006. Global topological features of cancer proteins in the human interactome. Bioinformatics 22(18): 2291–2297. doi:10.1093/bioinformatics/btl390. PMID: 16844706.
Palla, G., I. Derenyi, I. Farkas, and T. Vicsek. 2005. Uncovering the overlapping community structure of complex networks in nature and society. Nature 435(7043): 814–818. doi:10.1038/nature03607. PMID: 15944704.
Jou, I.M., C.F. Lin, K.J. Tsai, and S.J. Wei. 2013. Macrophage-mediated inflammatory disorders. Mediators of Inflammation 2013: 316482. doi:10.1155/2013/316482. PMID: 23843681.
Edwards, J.P., X. Zhang, K.A. Frauwirth, and D.M. Mosser. 2006. Biochemical and functional characterization of three activated macrophage populations. Journal of Leukocyte Biology 80(6): 1298–1307. doi:10.1189/jlb.0406249. PMID: 16905575.
Barabasi, A.L., and Z.N. Oltvai. 2004. Network biology: Understanding the cell’s functional organization. Nature Reviews Genetics 5(2): 101–113. doi:10.1038/nrg1272. PMID: 14735121.
Lim, J., T. Hao, C. Shaw, A.J. Patel, G. Szabo, J.F. Rual, C.J. Fisk, et al. 2006. A protein-protein interaction network for human inherited ataxias and disorders of Purkinje cell degeneration. Cell 125(4): 801–814. doi:10.1016/j.cell.2006.03.032. PMID: 16713569.
Ihmels, J., G. Friedlander, S. Bergmann, O. Sarig, Y. Ziv, and N. Barkai. 2002. Revealing modular organization in the yeast transcriptional network. Nature Genetics 31(4): 370–377. doi:10.1038/ng941. PMID: 12134151.
Newman, M.E. 2006. Modularity and community structure in networks. Proceedings of the National Academy of Sciences of the United States of America 103(23): 8577–8582. doi:10.1073/pnas.0601602103. PMID: 16723398.
Hoffmann, A., G. Natoli, and G. Ghosh. 2006. Transcriptional regulation via the NF-kappaB signaling module. Oncogene 25(51): 6706–6716. doi:10.1038/sj.onc.1209933. PMID: 17072323.
Kurata, H., H. El-Samad, R. Iwasaki, H. Ohtake, J.C. Doyle, I. Grigorova, C.A. Gross, and M. Khammash. 2006. Module-based analysis of robustness tradeoffs in the heat shock response system. PLoS Computational Biology 2(7): e59. doi:10.1371/journal.pcbi.0020059. PMID: 16863396.
Xue, D., W. Zhang, Y. Zhang, H. Wang, B. Zheng, and X. Shi. 2006. Adjusting effects of baicalin for nuclear factor-kappaB and tumor necrosis factor-alpha on rats with caerulein-induced acute pancreatitis. Mediators of Inflammation 2006(5): 26295. doi:10.1155/MI/2006/26295. PMID: 17392571.
Chen, L.F., and W.C. Greene. 2004. Shaping the nuclear action of NF-kappaB. Nature Reviews Molecular Cell Biology 5(5): 392–401. doi:10.1038/nrm1368. PMID: 15122352.
Blinman, T.A., I. Gukovsky, M. Mouria, V. Zaninovic, E. Livingston, S.J. Pandol, and A.S. Gukovskaya. 2000. Activation of pancreatic acinar cells on isolation from tissue: Cytokine upregulation via p38 MAP kinase. American Journal of Physiology-Cell Physiology 279(6): C1993–C2003. PMID: 11078716.
Rakonczay Jr., Z., K. Jarmay, J. Kaszaki, Y. Mandi, E. Duda, P. Hegyi, I. Boros, J. Lonovics, and T. Takacs. 2003. NF-kappaB activation is detrimental in arginine-induced acute pancreatitis. Free Radical Biology and Medicine 34(6): 696–709. doi:10.1016/S0891-5849(02)01373-4. PMID: 12633747.
Liu, H.S., C.E. Pan, Q.G. Liu, W. Yang, and X.M. Liu. 2003. Effect of NF-kappaB and p38 MAPK in activated monocytes/macrophages on pro-inflammatory cytokines of rats with acute pancreatitis. World Journal of Gastroenterology 9(11): 2513–2518. PMID: 14606087.
Li, X., Z. Li, Z. Zheng, Y. Liu, and X. Ma. 2007. Virulizin, a novel immunotherapy agent, stimulates TNFalpha expression in monocytes/macrophages in vitro and in vivo. International Immunopharmacology 7(10): 1350–1359. doi:10.1016/j.intimp.2007.06.001. PMID: 17673150.
Ma, Z.H., Q.Y. Ma, L.C. Wang, H.C. Sha, S.L. Wu, and M. Zhang. 2005. Effect of resveratrol on peritoneal macrophages in rats with severe acute pancreatitis. Inflammation Research 54(12): 522–527. doi:10.1007/s00011-005-1388-z. PMID: 16389574.
Liang, T., T.F. Liu, D.B. Xue, B. Sun, and L.J. Shi. 2008. Different cell death modes of pancreatic acinar cells on macrophage activation in rats. Chinese Medical Journal 121(19): 1920–1924. PMID: 19080125.
Gutierrez, P.T., E. Folch-Puy, O. Bulbena, and D. Closa. 2008. Oxidised lipids present in ascitic fluid interfere with the regulation of the macrophages during acute pancreatitis, promoting an exacerbation of the inflammatory response. Gut 57(5): 642–648. doi:10.1136/gut.2007.127472. PMID: 18203805.
Li, X., Z. Li, Z. Zheng, Y. Liu, and X. Ma. 2013. Unfractionated heparin ameliorates lipopolysaccharide-induced lung inflammation by downregulating nuclear factor-kappaB signaling pathway. Inflammation 36(6): 1201–1218. doi:10.1007/s10753-013-9656-5. PMID: 23690274.
Lee, J.W., M.S. Lee, T.H. Kim, H.J. Lee, S.S. Hong, Y.H. Noh, B.Y. Hwang, J.S. Ro, and J.T. Hong. 2007. Inhibitory effect of inflexinol on nitric oxide generation and iNOS expression via inhibition of NF-kappaB activation. Mediators of Inflammation 2007: 93148. doi:10.1155/2007/93148. PMID: 17541474.
Wadleigh, D.J., S.T. Reddy, E. Kopp, S. Ghosh, and H.R. Herschman. 2000. Transcriptional activation of the cyclooxygenase-2 gene in endotoxin-treated RAW 264.7 macrophages. Journal of Biological Chemistry 275(9): 6259–6266. doi:10.1074/jbc.275.9.6259. PMID: 10692422.
Jobin, C., and R.B. Sartor. 2000. The I kappa B/NF-kappa B system: a key determinant of mucosalinflammation and protection. American Journal of Physiology-Cell Physiology 278(3): C451–C462. PMID: 10712233.
Huang, L.Y., P. Chen, L.X. Xu, Y.F. Zhou, Y.P. Zhang, and Y.Z. Yuan. 2012. Fractalkine upregulates inflammation through CX3CR1 and the Jak-Stat pathway in severe acute pancreatitis rat model. Inflammation 35(3): 1023–1030. doi:10.1007/s10753-011-9406-5. PMID: 22213034.
Jung, H.J., S.J. Kim, W.K. Jeon, B.C. Kim, K. Ahn, K. Kim, Y.M. Kim, E.H. Park, and C.J. Lim. 2011. Anti-inflammatory activity of n-propyl gallate through down-regulation of NF-kappaB and JNK pathways. Inflammation 34(5): 352–361. doi:10.1007/s10753-010-9241-0. PMID: 20689985.
Gu, J., H. Zhang, L. Chen, S. Xu, G. Yuan, and X. Xu. 2011. Drug-target network and polypharmacology studies of a Traditional Chinese Medicine for type II diabetes mellitus. Computational Biology and Chemistry 35(5): 293–297. doi:10.1016/j.compbiolchem.2011.07.003. PMID: 22000800.
Zimmermann, G.R., J. Lehar, and C.T. Keith. 2007. Multi-target therapeutics: When the whole is greater than the sum of the parts. Drug Discovery Today 12(1–2): 34–42. doi:10.1016/j.drudis.2006.11.008. PMID: 17198971.
Borisy, A.A., P.J. Elliott, N.W. Hurst, M.S. Lee, J. Lehar, E.R. Price, G. Serbedzija, et al. 2003. Systematic discovery of multicomponent therapeutics. Proceedings of the National Academy of Sciences of the United States of America 100(13): 7977–7982. doi:10.1073/pnas.1337088100. PMID: 12799470.
Jingmin, O., Z. Xiping, W. Chun, Y. Ping, and Y. Qian. 2012. Study of dexamethasone, baicalin and octreotide on brain injury of rats with severe acute pancreatitis. Inflammation Research 61(3): 265–275. doi:10.1007/s00011-011-0408-4. PMID: 22166920.
Xiping, Z., P. Yan, H. Xinmei, F. Guanghua, M. Meili, N. Jie, and Z. Fangjie. 2010. Effects of dexamethasone and Salvia miltiorrhizae on the small intestine and immune organs of rats with severe acute pancreatitis. Inflammation 33(4): 259–266. doi:10.1007/s10753-010-9180-9. PMID: 20127399.
Makela, A., T. Kuusi, and T. Schroder. 1997. Inhibition of serum phospholipase-A2 in acute pancreatitis by pharmacological agents in vitro. Scandinavian Journal of Clinical and Laboratory Investigation 57(5): 401–407. doi:10.3109/00365519709084587. PMID: 9279965.
Criddle, D.N., S. Gillies, H.K. Baumgartner-Wilson, M. Jaffar, E.C. Chinje, S. Passmore, M. Chvanov, et al. 2006. Menadione-induced reactive oxygen species generation via redox cycling promotes apoptosis of murine pancreatic acinar cells. Journal of Biological Chemistry 281(52): 40485–40492. doi:10.1074/jbc.M607704200. PMID: 17088248.
Ju, K.D., J.W. Lim, K.H. Kim, and H. Kim. 2011. Potential role of NADPH oxidase-mediated activation of Jak2/Stat3 and mitogen-activated protein kinases and expression of TGF-beta1 in the pathophysiology of acute pancreatitis. Inflammation Research 60(8): 791–800. doi:10.1007/s00011-011-0335-4. PMID: 21509626.
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (81370566).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Wang, Y., Lu, M. et al. Modular Analysis of Bioinformatics Demonstrates a Critical Role for NF-κB in Macrophage Activation. Inflammation 37, 1240–1253 (2014). https://doi.org/10.1007/s10753-014-9851-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-014-9851-z