Abstract
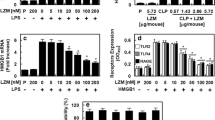
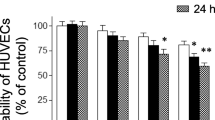
As a late mediator of inflammation, the high-mobility group box 1 protein (HMGB1) plays a key role in the inflammatory responses to tissue injury and infection by inducing and extending the production of proinflammatory cytokines. It has been observed that lower concentration thrombin mediates anti-inflammatory activities. The aim of this study was to investigate whether lower concentration thrombin could modulate HMGB1 expression and could inhibit HMGB1-mediated inflammatory responses in human umbilical vein endothelial cells (HUVECs). Here, results showed that lower concentration thrombin or thrombin receptor agonist peptide inhibits lipopolysaccharide-induced HMGB1 release from HUVECs. And lower concentration thrombin has inhibitory effects not only on the expression of cell adhesion molecules but also on neutrophils adhesion and migration toward HUVECs in response to HMGB1. Interestingly, the HMGB1-induced nuclear factor kappa B activation and tumor necrosis factor-alpha release from HUVECs were inhibited by lower concentration thrombin. Given these results, lower concentration thrombin could be a strong candidate as a therapeutic agent for various systemic inflammatory diseases.




Similar content being viewed by others
REFERENCES
Wang, H., O. Bloom, M. Zhang, et al. 1999. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285: 248–251.
Muller, S., P. Scaffidi, B. Degryse, et al. 2001. New EMBO members’ review: The double life of HMGB1 chromatin protein: Architectural factor and extracellular signal. EMBO Journal 20: 4337–4340.
Harris, H.E., and A. Raucci. 2006. Alarmin(g) news about danger: Workshop on innate danger signals and HMGB1. EMBO Reports 7: 774–778.
DeMarco, R.A., M.P. Fink, and M.T. Lotze. 2005. Monocytes promote natural killer cell interferon gamma production in response to the endogenous danger signal HMGB1. Molecular Immunology 42: 433–444.
Scaffidi, P., T. Misteli, and M.E. Bianchi. 2002. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418: 191–195.
Andersson, U., H. Wang, K. Palmblad, et al. 2000. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. The Journal of Experimental Medicine 192: 565–570.
Fiuza, C., M. Bustin, S. Talwar, et al. 2003. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood 101: 2652–2660.
Park, J.S., D. Svetkauskaite, Q. He, et al. 2004. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. Journal of Biological Chemistry 279: 7370–7377.
Vallet, B. 2003. Bench-to-bedside review: Endothelial cell dysfunction in severe sepsis: A role in organ dysfunction? Critical Care 7: 130–138.
Ross, R. 1993. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature 362: 801–809.
Wu, K.K., and P. Thiagarajan. 1996. Role of endothelium in thrombosis and hemostasis. Annual Review of Medicine 47: 315–331.
Yamagami, H., S. Yamagami, T. Inoki, S. Amano, and K. Miyata. 2003. The effects of proinflammatory cytokines on cytokine-chemokine gene expression profiles in the human corneal endothelium. Investigative Ophthalmology & Visual Science 44: 514–520.
Zeuke, S., A.J. Ulmer, S. Kusumoto, H.A. Katus, and H. Heine. 2002. TLR4-mediated inflammatory activation of human coronary artery endothelial cells by LPS. Cardiovascular Research 56: 126–134.
Chen, C.C., and A.M. Manning. 1995. Transcriptional regulation of endothelial cell adhesion molecules: A dominant role for NF-kappa B. Agents and Actions. Supplements 47: 135–141.
Coughlin, S.R. 2000. Thrombin signalling and protease-activated receptors. Nature 407: 258–264.
Macfarlane, S.R., M.J. Seatter, T. Kanke, G.D. Hunter, and R. Plevin. 2001. Proteinase-activated receptors. Pharmacological Reviews 53: 245–282.
Ossovskaya, V.S., and N.W. Bunnett. 2004. Protease-activated receptors: Contribution to physiology and disease. Physiological Reviews 84: 579–621.
Steinhoff, M., J. Buddenkotte, V. Shpacovitch, et al. 2005. Proteinase-activated receptors: Transducers of proteinase-mediated signaling in inflammation and immune response. Endocrine Reviews 26: 1–43.
Coughlin, S.R. 2005. Protease-activated receptors in hemostasis, thrombosis and vascular biology. Journal of Thrombosis and Haemostasis 3: 1800–1814.
Bae, J.S., Y.U. Kim, M.K. Park, and A.R. Rezaie. 2009. Concentration dependent dual effect of thrombin in endothelial cells via Par-1 and Pi3 kinase. Journal of Cellular Physiology 219: 744–751.
Bae, J.S., L. Yang, C. Manithody, and A.R. Rezaie. 2007. The ligand occupancy of endothelial protein C receptor switches the protease-activated receptor 1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood 110: 3909–3916.
Feistritzer, C., and M. Riewald. 2005. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood 105: 3178–3184.
Bae, J.S. 2011. Inhibitory effect of thrombin on the expression of secretory group IIA phospholipase A. Journal of Cellular Biochemistry 112: 2502–2507.
Bae, J.S., and A.R. Rezaie. 2011. Activated protein C inhibits high mobility group box 1 signaling in endothelial cells. Blood 118: 3952–3959.
Akeson, A.L., and C.W. Woods. 1993. A fluorometric assay for the quantitation of cell adherence to endothelial cells. Journal of Immunological Methods 163: 181–185.
Che, W., N. Lerner-Marmarosh, Q. Huang, et al. 2002. Insulin-like growth factor-1 enhances inflammatory responses in endothelial cells: Role of Gab1 and MEKK3 in TNF-alpha-induced c-Jun and NF-kappaB activation and adhesion molecule expression. Circulation Research 90: 1222–1230.
Mullins, G.E., J. Sunden-Cullberg, A.S. Johansson, et al. 2004. Activation of human umbilical vein endothelial cells leads to relocation and release of high-mobility group box chromosomal protein 1. Scandinavian Journal of Immunology 60: 566–573.
Yang, J., C. Huang, H. Jiang, and J. Ding. 2010. Statins attenuate high mobility group box-1 protein induced vascular endothelial activation: A key role for TLR4/NF-kappaB signaling pathway. Molecular and Cellular Biochemistry 345: 189–195.
Treutiger, C.J., G.E. Mullins, A.S. Johansson, et al. 2003. High mobility group 1 B-box mediates activation of human endothelium. Journal of Internal Medicine 254: 375–385.
Javaid, K., A. Rahman, K.N. Anwar, R.S. Frey, R.D. Minshall, and A.B. Malik. 2003. Tumor necrosis factor-alpha induces early-onset endothelial adhesivity by protein kinase Czeta-dependent activation of intercellular adhesion molecule-1. Circulation Research 92: 1089–1097.
Lockyer, J.M., J.S. Colladay, W.L. Alperin-Lea, T. Hammond, and A.J. Buda. 1998. Inhibition of nuclear factor-kappaB-mediated adhesion molecule expression in human endothelial cells. Circulation Research 82: 314–320.
Park, J.S., F. Gamboni-Robertson, Q. He, et al. 2006. High mobility group box 1 protein interacts with multiple Toll-like receptors. American Journal of Physiology. Cell Physiology 290: C917–C924.
Ossovskaya, V.S., N.W. Bunnett, L. de Garavilla, et al. 2004. Protease-activated receptors: Contribution to physiology and disease. Physiological Reviews 84: 579–621.
Shankar, R., C.A. de la Motte, E.J. Poptic, and P.E. DiCorleto. 1994. Thrombin receptor-activating peptides differentially stimulate platelet-derived growth factor production, monocytic cell adhesion, and E-selectin expression in human umbilical vein endothelial cells. Journal of Biological Chemistry 269: 13936–13941.
Bae, J.S., and A.R. Rezaie. 2008. Protease activated receptor 1 (PAR-1) activation by thrombin is protective in human pulmonary artery endothelial cells if endothelial protein C receptor is occupied by its natural ligand. Thrombosis and Haemostasis 100: 101–109.
Vu, T.K., D.T. Hung, V.I. Wheaton, and S.R. Coughlin. 1991. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 64: 1057–1068.
Zain, J., Y.Q. Huang, X. Feng, M.L. Nierodzik, J.J. Li, and S. Karpatkin. 2000. Concentration-dependent dual effect of thrombin on impaired growth/apoptosis or mitogenesis in tumor cells. Blood 95: 3133–3138.
Striggow, F., M. Riek, J. Breder, P. Henrich-Noack, K.G. Reymann, and G. Reiser. 2000. The protease thrombin is an endogenous mediator of hippocampal neuroprotection against ischemia at low concentrations but causes degeneration at high concentrations. Proceedings of the National Academy of Sciences of the United States of America 97: 2264–2269.
Ding, H.S., and J. Yang. 2010. High mobility group box-1 and cardiovascular diseases. Saudi Medical Journal 31: 486–489.
El Gazzar, M. 2007. HMGB1 modulates inflammatory responses in LPS-activated macrophages. Inflammation Research 56: 162–167.
Rouhiainen, A., J. Kuja-Panula, E. Wilkman, et al. 2004. Regulation of monocyte migration by amphoterin (HMGB1). Blood 104: 1174–1182.
Degryse, B., and M. de Virgilio. 2003. The nuclear protein HMGB1, a new kind of chemokine? FEBS Letters 553: 11–17.
Yang, H., M. Ochani, J. Li, et al. 2004. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proceedings of the National Academy of Sciences of the United States of America 101: 296–301.
Hortelano, S., R. Lopez-Fontal, P.G. Traves, et al. 2010. ILK mediates LPS-induced vascular adhesion receptor expression and subsequent leucocyte trans-endothelial migration. Cardiovascular Research 86: 283–292.
Dal-Secco, D., A. Freitas, M.A. Abreu, et al. 2010. Reduction of ICAM-1 expression by carbon monoxide via soluble guanylate cyclase activation accounts for modulation of neutrophil migration. Naunyn-Schmiedeberg’s Archives of Pharmacology 381: 483–493.
Park, J.S., J. Arcaroli, H.K. Yum, et al. 2003. Activation of gene expression in human neutrophils by high mobility group box 1 protein. American Journal of Physiology. Cell Physiology 284: C870–C879.
Langer, H.F., and T. Chavakis. 2009. Leukocyte–endothelial interactions in inflammation. Journal of Cellular and Molecular Medicine 13: 1211–1220.
Pardi, R., L. Inverardi, and J.R. Bender. 1992. Regulatory mechanisms in leukocyte adhesion: Flexible receptors for sophisticated travelers. Immunology Today 13: 224–230.
ACKNOWLEDGEMENT
These studies were supported by a National Research Foundation of Korea Grant funded by the Korean Government (2011-0003410 and 2011-0026695).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bae, JS. Effects of Lower Concentration Thrombin on High-Mobility Group Box 1 Protein-Mediated Inflammatory Responses. Inflammation 35, 1078–1086 (2012). https://doi.org/10.1007/s10753-011-9414-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-011-9414-5