Abstract
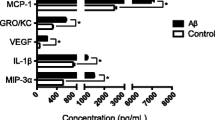
Age-related macular degeneration (AMD) is the predominant cause of irreversible blindness in the elderly population. Despite intensive basic and clinical research, its pathogenesis remains unclear. However, evidence suggests that immunological and inflammatory factors contribute to the pathogenesis of AMD. A newly identified cytokine, IL-33, appears to be an important pro-inflammatory cytokine promoting tissue inflammation. In this study, IL-33 was increased through amyloid-beta1–40 (Aβ1–40) stimulation and regulated inflammatory cytokines including IL-6, IL-8, IL-1β, and TNF-α secretion using different signaling pathways in retinal pigment epithelium (RPE) cells. Furthermore, ST2L, the important component of the IL-33 receptor, was significantly increased following recombinant human IL-33 stimulation in RPE cells. These findings suggest that IL-33-mediated inflammatory responses in RPE cells are involved in the pathogenesis of AMD. Greater understanding of the inflammatory effect of IL-33 and its role in RPE cells should aid the development of future clinical therapeutics and enable novel pharmacological approaches towards the prevention of AMD.






Similar content being viewed by others
References
Klein, R., T. Peto, A. Bird, and M.R. Vannewkirk. 2004. The epidemiology of age-related macular degeneration. American Journal of Ophthalmology 137: 486–495.
Kuehn, B.M. 2005. Gene discovery provides clues to cause of age-related macular degeneration. JAMA 293: 1841–1845.
Rodrigues, E.B. 2007. Inflammation in dry age-related macular degeneration. Ophthalmologica 221: 143–152.
Kijlstra, A., E. La Heij, and F. Hendrikse. 2005. Immunological factors in the pathogenesis and treatment of age-related macular degeneration. Ocular Immunology and Inflammation 13: 3–11.
Stolarski, B., M. Kurowska-Stolarska, P. Kewin, D. Xu, and F.Y. Liew. 2010. IL-33 exacerbates eosinophil-mediated airway inflammation. Journal of Immunology 185: 3472–3480.
Mato, N., M. Bando, H. Yamasawa, T. Hosono, Y. Mizushina, M. Sata, G. Ohki, and Y. Sugiyama. 2010. Role of IL-33 in bronchial asthma. Nihon Kokyūki Gakkai Zasshi 48: 419–425.
Prefontaine, D., J. Nadigel, F. Chouiali, S. Audusseau, A. Semlali, J. Chakir, J.G. Martin, and Q. Hamid. 2010. Increased IL-33 expression by epithelial cells in bronchial asthma. The Journal of Allergy and Clinical Immunology 125: 752–754.
Matsuyama, Y., H. Okazaki, H. Tamemoto, H. Kimura, Y. Kamata, K. Nagatani, T. Nagashima, M. Hayakawa, M. Iwamoto, T. Yoshio, et al. 2010. Increased levels of interleukin 33 in sera and synovial fluid from patients with active rheumatoid arthritis. Journal of Rheumatology 37: 18–25.
Mok, M.Y., F.P. Huang, W.K. Ip, Y. Lo, F.Y. Wong, E.Y. Chan, K.F. Lam, and D. Xu. 2010. Serum levels of IL-33 and soluble ST2 and their association with disease activity in systemic lupus erythematosus. Rheumatology (Oxford, England) 49: 520–527.
Bressler, S.B., M.G. Maguire, N.M. Bressler, and S.L. Fine. 1990. Relationship of drusen and abnormalities of the retinal pigment epithelium to the prognosis of neovascular macular degeneration. The Macular Photocoagulation Study Group. Archives of Ophthalmology 108: 1442–1447.
Vinding, T. 1990. Occurrence of drusen, pigmentary changes and exudative changes in the macula with reference to age-related macular degeneration. An epidemiological study of 1000 aged individuals. Acta Ophthalmol (Copenh) 68: 410–414.
Handa, J.T., N. Verzijl, H. Matsunaga, A. Aotaki-Keen, G.A. Lutty, J.M. te Koppele, T. Miyata, and L.M. Hjelmeland. 1999. Increase in the advanced glycation end product pentosidine in Bruch’s membrane with age. Investigative Ophthalmology and Visual Science 40: 775–779.
Pauleikhoff, D., S. Zuels, G.S. Sheraidah, J. Marshall, A. Wessing, and A.C. Bird. 1992. Correlation between biochemical composition and fluorescein binding of deposits in Bruch’s membrane. Ophthalmology 99: 1548–1553.
Anderson, D.H., K.C. Talaga, A.J. Rivest, E. Barron, G.S. Hageman, and L.V. Johnson. 2004. Characterization of beta amyloid assemblies in drusen: The deposits associated with aging and age-related macular degeneration. Experimental Eye Research 78: 243–256.
Dentchev, T., A.H. Milam, V.M. Lee, J.Q. Trojanowski, and J.L. Dunaief. 2003. Amyloid-beta is found in drusen from some age-related macular degeneration retinas, but not in drusen from normal retinas. Molecular Vision 9: 184–190.
Luibl, V., J.M. Isas, R. Kayed, C.G. Glabe, R. Langen, and J. Chen. 2006. Drusen deposits associated with aging and age-related macular degeneration contain nonfibrillar amyloid oligomers. The Journal of Clinical Investigation 116: 378–385.
Mullins, R.F., S.R. Russell, D.H. Anderson, and G.S. Hageman. 2000. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. The FASEB Journal 14: 835–846.
Ning, A., J. Cui, E. To, K.H. Ashe, and J. Matsubara. 2008. Amyloid-beta deposits lead to retinal degeneration in a mouse model of Alzheimer disease. Investigative Ophthalmology and Visual Science 49: 5136–5143.
Yoshida, T., K. Ohno-Matsui, S. Ichinose, T. Sato, N. Iwata, T.C. Saido, T. Hisatomi, M. Mochizuki, and I. Morita. 2005. The potential role of amyloid beta in the pathogenesis of age-related macular degeneration. The Journal of Clinical Investigation 115: 2793–2800.
Kurji, K.H., J.Z. Cui, T. Lin, D. Harriman, S.S. Prasad, L. Kojic, and J.A. Matsubara. 2010. Microarray analysis identifies changes in inflammatory gene expression in response to amyloid-beta stimulation of cultured human retinal pigment epithelial cells. Investigative Ophthalmology and Visual Science 51: 1151–1163.
Chackerian, A.A., E.R. Oldham, E.E. Murphy, J. Schmitz, S. Pflanz, and R.A. Kastelein. 2007. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. Journal of Immunology 179: 2551–2555.
Oboki, K., T. Ohno, N. Kajiwara, H. Saito, and S. Nakae. 2010. IL-33 and IL-33 receptors in host defense and diseases. Allergology International 59: 143–160.
Schmitz, J., A. Owyang, E. Oldham, Y. Song, E. Murphy, T.K. McClanahan, G. Zurawski, M. Moshrefi, J. Qin, X. Li, et al. 2005. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23: 479–490.
Anderson, D.H., R.F. Mullins, G.S. Hageman, and L.V. Johnson. 2002. A role for local inflammation in the formation of drusen in the aging eye. American Journal of Ophthalmology 134: 411–431.
Donoso, L.A., D. Kim, A. Frost, A. Callahan, and G. Hageman. 2006. The role of inflammation in the pathogenesis of age-related macular degeneration. Survey of Ophthalmology 51: 137–152.
Meda, L., P. Baron, E. Prat, E. Scarpini, G. Scarlato, M.A. Cassatella, and F. Rossi. 1999. Proinflammatory profile of cytokine production by human monocytes and murine microglia stimulated with beta-amyloid[25–35]. Journal of Neuroimmunology 93: 45–52.
Eikelenboom, P., E. van Exel, J.J. Hoozemans, R. Veerhuis, A.J. Rozemuller, and W.A. van Gool. 2010. Neuroinflammation—An early event in both the history and pathogenesis of Alzheimer’s disease. Neurodegenerative Diseases 7: 38–41.
He, F.Q., B.Y. Qiu, T.K. Li, Q. Xie, D.J. Cui, X.L. Huang, and H.T. Gan. 2011. Tetrandrine suppresses amyloid-beta-induced inflammatory cytokines by inhibiting NF-kappaB pathway in murine BV2 microglial cells. Int Immunopharmacol 11: 1220–1225.
Lindberg, C., M.L. Selenica, A. Westlind-Danielsson, and M. Schultzberg. 2005. Beta-amyloid protein structure determines the nature of cytokine release from rat microglia. Journal of Molecular Neuroscience 27: 1–12.
Liu, B., and J.S. Hong. 2003. Role of microglia in inflammation-mediated neurodegenerative diseases: Mechanisms and strategies for therapeutic intervention. Journal of Pharmacology and Experimental Therapeutics 304: 1–7.
Veerhuis, R., R.S. Boshuizen, M. Morbin, G. Mazzoleni, J.J. Hoozemans, J.P. Langedijk, F. Tagliavini, J.P. Langeveld, and P. Eikelenboom. 2005. Activation of human microglia by fibrillar prion protein-related peptides is enhanced by amyloid-associated factors SAP and C1q. Neurobiology of Disease 19: 273–282.
Beltran, C.J., L.E. Nunez, D. Diaz-Jimenez, N. Farfan, E. Candia, C. Heine, F. Lopez, M.J. Gonzalez, R. Quera, and M.A. Hermoso. 2010. Characterization of the novel ST2/IL-33 system in patients with inflammatory bowel disease. Inflammatory Bowel Diseases 16: 1097–1107.
Seidelin, J.B., J.T. Bjerrum, M. Coskun, B. Widjaya, B. Vainer, and O.H. Nielsen. 2010. IL-33 is upregulated in colonocytes of ulcerative colitis. Immunology Letters 128: 80–85.
Manetti, M., L. Ibba-Manneschi, V. Liakouli, S. Guiducci, A.F. Milia, G. Benelli, A. Marrelli, M.L. Conforti, E. Romano, R. Giacomelli, et al. 2010. The IL1-like cytokine IL33 and its receptor ST2 are abnormally expressed in the affected skin and visceral organs of patients with systemic sclerosis. Annals of the Rheumatic Diseases 69: 598–605.
Marvie, P., M. Lisbonne, A. L’Helgoualc’h, M. Rauch, B. Turlin, L. Preisser, K. Bourd-Boittin, N. Theret, H. Gascan, C. Piquet-Pellorce, et al. 2010. Interleukin-33 overexpression is associated with liver fibrosis in mice and humans. Journal of Cellular and Molecular Medicine 14: 1726–1739.
Moulin, D., O. Donze, D. Talabot-Ayer, F. Mezin, G. Palmer, and C. Gabay. 2007. Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine 40: 216–225.
Iikura, M., H. Suto, N. Kajiwara, K. Oboki, T. Ohno, Y. Okayama, H. Saito, S.J. Galli, and S. Nakae. 2007. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Laboratory Investigation 87: 971–978.
Funakoshi-Tago, M., K. Tago, Y. Sato, S. Tominaga, and T. Kasahara. 2011. JAK2 is an important signal transducer in IL-33-induced NF-kappaB activation. Cellular Signalling 23: 363–370.
Yagami, A., K. Orihara, H. Morita, K. Futamura, N. Hashimoto, K. Matsumoto, H. Saito, and A. Matsuda. 2010. IL-33 mediates inflammatory responses in human lung tissue cells. Journal of Immunology 185: 5743–5750.
Kunes, P., Z. Holubcova, M. Kolackova, and J. Krejsek. 2010. The counter-regulation of atherogenesis: A role for interleukin-33. Acta Medica (Hradec Králové) 53: 125–129.
Dinarello, C.A. 2009. Immunological and inflammatory functions of the interleukin-1 family. Annual Review of Immunology 27: 519–550.
O’Neill, L.A. 2008. The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunological Reviews 226: 10–18.
Suzukawa, M., M. Iikura, R. Koketsu, H. Nagase, C. Tamura, A. Komiya, S. Nakae, K. Matsushima, K. Ohta, K. Yamamoto, et al. 2008. An IL-1 cytokine member, IL-33, induces human basophil activation via its ST2 receptor. Journal of Immunology 181: 5981–5989.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, XC., Liu, XF., Jian, CX. et al. IL-33 Is Induced by Amyloid-β Stimulation and Regulates Inflammatory Cytokine Production in Retinal Pigment Epithelium Cells. Inflammation 35, 776–784 (2012). https://doi.org/10.1007/s10753-011-9379-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-011-9379-4