Abstract
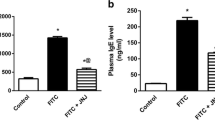
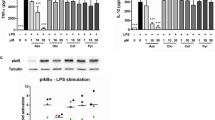
Macrolide antibiotics inhibit the secretion of Th1 cytokines while their effects on the release of Th2 cytokines are variable. We investigated molecular and cellular markers of Th1- and Th2-mediated inflammatory mechanisms and the anti-inflammatory activity of azithromycin and clarithromycin in phorbol 12-myristate 13-acetate (PMA) and oxazolone (OXA)-induced skin inflammation. Dexamethasone (50 μg/ear), azithromycin, and clarithromycin (500 μg/ear) reduced TNF-α and interleukin (IL)-1β concentration in ear tissue by inhibiting inflammatory cell accumulation in PMA-induced inflammation. In OXA-induced early delayed-type hypersensitivity (DTH), the macrolides (2 mg/ear) and dexamethasone (25 μg/ear) reduced ear tissue inflammatory cell infiltration and secretion of IL-4 while clarithromycin also decreased IFN-γ concentration. Macrolides showed better activity when administered after the challenge. In OXA-induced chronic DTH, azithromycin (1 mg/ear) reduced the number of ear tissue mast cells and decreased the concentration of IL-4 in ear tissue and of immunoglobulin (Ig)E in serum. Clarithromycin (1 mg/ear) reduced serum IgE concentration, possibly by a mechanism independent of IL-4, while both macrolides attenuated mast cell degranulation. In conclusion, azithromycin and clarithromycin attenuate pro-inflammatory cytokine production and leukocyte infiltration during innate immune reactions, while selectively affecting Th2 rather than Th1 immunity in DTH reactions.











Similar content being viewed by others
REFERENCES
Zhanel, G.G., M. Dueck, D.J. Hoban, L.M. Vercaigne, J.M. Embil, A.S. Gin, and J.A. Karlowsky. 2001. Review of macrolides and ketolides: focus on respiratory tract infections. Drugs 61: 443–498.
Amsden, G.W. 2005. Anti-inflammatory effects of macrolides—an underappreciated benefit in the treatment of the community acquired respiratory tract infections and chronic inflammatory pulmonary conditions? The Journal of Antimicrobial Chemotherapy 55: 10–21.
Čuli, O., V. Erakovi, and M.J. Parnham. 2001. Anti-inflammatory effects of macrolide antibiotics. European Journal of Pharmacology 429: 209–229.
Labro, M.T. 2004. Cellular and molecular effects of macrolides on leukocyte function. Current Pharmaceutical Design 10: 3067–3080.
Gladue, R.P., G.M. Bright, R.E. Isaacson, and M.F. Newborg. 1989. In vitro and in vivo uptake of azithromycin (CP-62, 993) by phagocytic cells: possible mechanism of delivery and release at sites of infection. Antimicrobial Agents and Chemotherapy 33: 277–282.
Wildfeuer, A., H. Laufen, D. Muller-Wening, and O. Haferkamp. 1989. Interaction of azithromycin and human phagocytic cells. Uptake of the antibiotic and the effect on the survival of ingested bacteria in phagocytes. Drug Research/Arzneimittelforschung 39: 755–758.
Wildfeuer, A., H. Laufen, and T. Zimmermann. 1996. Uptake of azithromycin by various cells and its intracellular activity under in vivo conditions. Antimicrobial Agents and Chemotherapy 40: 75–79.
Čuli, O., V. Erakovi, I. Cepelak, K. Bariši, K. Brajša, Ž. Ferenči, R. Galovi, I. Glojnari, Z. Manojlovi, V. Muni, R. Novak-Mirceti, V. Paviči-Beljak, M. Sui, M. Veljača, T. Žani-Grubiši, and M.J. Parnham. 2002. Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects. European Journal of Pharmacology 450: 277–289.
Ianaro, A., A. Ialenti, P. Maffia, L. Sautebin, L. Rombola, R. Carnuccio, T. Iuvone, F. D'Acquisto, and M. Di Rosa. 2000. Anti-inflammatory activity of macrolide antibiotics. The Journal of Pharmacology and Experimental Therapeutics 292: 156–163.
Khan, A.A., T.R. Slifer, F.G. Araujo, and J.S. Remington. 1999. Effect of clarithromycin and azithromycin on production of cytokines by human monocytes. International Journal of Antimicrobial Agents 11: 121–132.
Terao, H., K. Asano, K. Kanai, Y. Kyo, S. Watanabe, T. Hisamitsu, and H. Suzaki. 2003. Suppressive activity of macrolide antibiotics on nitric oxide production by lipopolysaccharide stimulation in mice. Mediator Inflammation 12: 195–202.
Sanz, M.J., Y.N. Nabah, M. Cerda-Nicolas, J.E. O'Connor, A.C. Issekutz, J. Cortijo, and E.J. Morcillo. 2005. Erythromycin exerts in vivo anti-inflammatory activity downregulating cell adhesion molecule expression. British Journal of Pharmacology 144: 190–201.
Kita, E., M. Sawaki, K. Mikasa, K. Hamada, S. Takeuchi, K. Maeda, and N. Narita. 1993. Alterations of host response by a long-term treatment of roxithromycin. The Journal of Antimicrobial Chemotherapy 32: 285–294.
Azuma, A., T. Furuta, T. Enomoto, Y. Hashimoto, K. Uematsu, N. Nukariya, A. Murata, and S. Kudoh. 1998. Preventive effect of erythromycin on experimental bleomycin-induced acute lung injury in rats. Thorax 53: 186–189.
Pinto, L.A., C. Camozzato, M. Avozani, D. Cantarelli Machado, M.H. Jones, R. Tetelbom Stein, and P.M. Condessa Pitrez. 2004. Effect of Clarithromycin on the cell profile of bronchoalveolar lavage fluid in mice with neutrophil-predominant lung disease. Revista do Hospital das Clinicas, Faculdade de Medicina da Universidade de Sao Paulo 59: 99–103.
Suzaki, H., K. Asano, S. Ohki, K. Kanai, T. Mizutani, and T. Hisamitsu. 1999. Suppressive activity of a macrolide antibiotic, roxithromycin, on pro-inflammatory cytokine production in vitro and in vivo. Mediators of Inflammation 8: 199–204.
Tamaoki, J., J. Nakata, E. Tagaya, and K. Konno. 1996. Effects of roxithromycin and erythromycin in interleukin 8-induced neutrophil recruitment and goblet cell secretion in guinea pig tracheas. Antimicrobial Agents and Chemotherapy 40: 1726–1728.
Tkalčevi, V.I., B. Bošnjak, B. Hrvači, M. Bosnar, N. Marjanovi, Ž. Ferenči, K. Šitum, O. Čuli, M.J. Parnham, and V. Erakovi. 2006. Anti-inflammatory activity of azithromycin attenuates the effects of lipopolysaccharide administration in mice. European Journal of Pharmacology 539: 131–138.
Tkalčevi, V.I., B. Bošnjak, I. Pašali, B. Hrvači, K. Šitum, M.D. Kramari, I. Glojnari, and V.E. Haber. 2008. The anti-inflammatory activity of clarithromycin inhibits TNFα production and prolongs survival following lipopolysaccharide administration in mice. International Journal of Antimicrobial Agents 32: 195–196.
Agen, C., R. Danesi, C. Blandizzi, M. Costa, B. Stacchini, P. Favini, and M. Del Tacca. 1993. Macrolide antibiotics as anti-inflammatory agents: Roxythromycin in unexpected role. Agents and Actions 38: 85–90.
Scaglione, F., and G. Rossoni. 1998. Comparative anti-inflammatory effects of roxithromycin, azithromycin and clarithromycin. The Journal of Antimicrobial Chemotherapy 41: 47–50.
Tarayre, J.P., M. Aliaga, M. Barbara, G. Villanova, R. Ballester, J. Tisne-Versailles, and J.P. Couzinier. 1987. Cutaneously applied erythromycin base reduces various types of inflammatory reactions in mouse ear. International Journal of Tissue Reactions 9: 77–85.
Hashimoto, Y., Y. Kaneda, N. Takahashi, S. Akashi, I. Arai, and S. Nakaike. 2003. Clarithromycin inhibits the development of dermatitis in NC/Nga mice. Chemotherapy 49: 222–228.
Napoletano, M., E. Moriggi, A. Mereu, F. Ornaghi, G. Morazzoni, R. Longoni, C. Riva, L. Pacchetti, and F. Pellacini. 2004. Macrolide compounds endowed with anti-inflammatory activity. PCT/EP2003/012071. International publication number WO 2004/013153 A2.
Webb, E.F., M.N. Tzimas, S.J. Newsholme, and D.E. Griswold. 1998. Intralesional cytokines in chronic oxazolone-induced contact sensitivity suggest role for tumor necrosis factor α and interleukin-4. The Journal of Investigative Dermatology 111: 86–92.
Young, J.M., and L.M. De Young. 1989. Cutaneous models of inflammation for the evaluation of topical and systemic pharmacological agents. In Pharmacological methods in the control of inflammation, 1st ed, ed. J.Y. Chang and A.J. Lewis, 215–231. New York: Alan R. Liss, Inc.
Takeshita, K., I. Yamagishi, M. Harada, S. Otumo, T. Nakagawa, and Y. Mizushima. 1989. Immunological and anti-inflammatory effects of clarithromycin: inhibition of interleukin 1 production of murine peritoneal macrophages. Drugs under Experimental and Clinical Research 15: 527–533.
Bosnar, M., B. Bošnjak, S. Čuži, B. Hrvači, N. Marjanovi, I. Glojnari, O. Čuli, M.J. Parnham, and V.E. Haber. 2009. Azithromycin and clarithromycin inhibit lipopolysaccharide-induced murine pulmonary neutrophilia mainly through effects on macrophage-derived granulocyte-macrophage colony-stimulating factor and interleukin-1β. Journal of Pharmacology and Experimental Therapeutics 331: 104–113.
Fujii, Y., H. Takeuchi, K. Tanaka, S. Sakuma, Y. Ohkubo, and S. Mutoh. 2002. Effects of FK506 (tacrolimus hydrate) on chronic oxazolone-induced dermatitis in rats. European Journal of Pharmacology 456: 115–121.
Zunic, M., G.M. Bahr, G.C. Mudde, J.G. Meingassner, and C. Lam. 1998. MDP(Lysyl)GDP, a nontoxic muramyl dipeptide derivative, inhibits cytokine production by activated macrophages and protects mice from phorbol ester- and oxazolone-induced inflammation. The Journal of Investigative Dermatology 111: 77–82.
Elder, D., R. Elenitsas, C. Jaworsky, and B. Johnson Jr. (eds.). 1997. Lever's histopathology of the skin, 8th ed. Philadelphia: Lippincott–Raven Publishers.
Gregory, G.D., S.S. Raju, S. Winandy, and M.A. Brown. 2006. Mast cell IL-4 expression is regulated by Ikaros and influences encephalitogenic Th1 responses in EAE. The Journal of Clinical Investigation 116: 1327–1336.
Kashiwada, M., D.M. Levy, L. McKeag, K. Murray, A.J. Schroder, S.M. Canfield, G. Traver, and P.B. Rothman. 2010. IL-4-induced transcription factor NFIL3/E4BP4 controls IgE class switching. Proceedings of the National Academy of Sciences 107: 821–826.
Morikawa, K., J. Zhang, M. Nonaka, and S. Morikawa. 2002. Modulatory effect of macrolide antibiotics on the Th1- and Th2-type cytokine production. International Journal of Antimicrobial Agents 19: 53–9.
Kawazu, K., M. Kurokawa, K. Asano, A. Mita, and M. Adachi. 2000. Suppressive activity of a macrolide antibiotic, roxithromycin on co-stimulatory molecule expression on mouse splenocytes in vivo. Mediators of Inflammation 9: 39–43.
Hrvači, B., B. Bošnjak, M. Bosnar, Ž. Ferenči, I. Glojnari, and V. Erakovi Haber. 2009. Clarithromycin suppresses airway hyperresponsiveness and inflammation in mouse models of asthma. European Journal of Pharmacology 616: 236–243.
Van der Pouw Kraan, C.T.M., R.C. Aalberse, and L.A. Aarden. 1994. IgE production in atopic patients is not related to IL-4 production. Clinical and Experimental Immunology 97: 254–259.
Murphy, B.S., V. Sundareshan, T.J. Cory, T.J. Cory, D. Hayes Jr., M.I. Anstead, and D.J. Feola. 2008. Azithromycin alters macrophage phenotype. The Journal of Antimicrobial Chemotheraphy 61: 554–60.
ACKNOWLEDGMENTS
This work was supported by GlaxoSmithKline Research Centre Zagreb Ltd. The authors wish to thank I. Glojnari, Ph.D. for team leading, and Ms. S. Skender and I. ubela and Mr. H. Poduška for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ivetić Tkalčević, V., Čužić, S., Dominis Kramarić, M. et al. Topical Azithromycin and Clarithromycin Inhibit Acute and Chronic Skin Inflammation in Sensitized Mice, with Apparent Selectivity for Th2-Mediated Processes in Delayed-Type Hypersensitivity. Inflammation 35, 192–205 (2012). https://doi.org/10.1007/s10753-011-9305-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-011-9305-9