Abstract
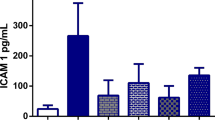
Mitochondria and lysosomes were evaluated by assessment of changes in activity of selected enzymes: lactate dehydrogenase (LDH), succinate dehydrogenase (SDH), adenosinetriphosphatase (ATPase), acid phosphatase (AcPase) and β-glucuronidase (BG) in rats under profound hypoxia induced by endotoxemic shock. The study was conducted on adult male Wistar rats. The animals formed the following four groups of 15 rats each: control animals (C);—rats receiving intraperitonally O2/O3 (CO), rats receiving of Escherichia coli toxin (LPS) (CL); rats receiving LPS plus oxygen–ozone mixture (OL). Histoenzymatic examinations of liver, kidney, lungs, and heart muscle were performed. Lipopolysaccharide suppressed activities of all the enzymes except for LDH, the activity of which as high as a fourfold increase. The results demonstrated potent, stabilizing and regenerative effects of ozone therapy on body enzymatic processes in course of induced endotoxemic shock in rats, which might prove to be of clinical significance.

















Similar content being viewed by others
REFERENCES
Bone, R. C. 1994. Sepsis and its complications: the clinical problem. Crit. Care Med. 22:S8–S11.
Figureoa, J. A., D. Yee, and W. L. McGuire. 1993. Prognostic indicators in early breast cancer. Am. J. Med. Sci. 305:176–182.
Thijs, L. G., J. P. de Boer, M. C. de Groot, and C. E. Hack. 1993. Coagulation disorders in septic shock. Intensive Care Med. 19:S8–S15.
Thijs, L. G., A. B. Groeneveld, and C. E. Hack. 1996. Multiple organ failure in septic shock. Curr. Top Microbiol. Immunol. 216:209–237.
Barriere, S. L., and B. J. Guglielmo. 1992. Gram-negative sepsis, the sepsis syndrome, and the role of antiendotoxin monoclonal antibodies. Clin. Pharm. 11:223–235.
Heng, H., R. B. Rucker, J. Crotty, and M. A. Dubick. 1987. The effects of ozone on lung, heart, and liver superoxide dismutase and glutathione peroxidase activities in the protein-deficient rat. Toxicol. Lett. 38:225–237.
Bone, R. C. 1994. Gram-positive organisms and sepsis. Arch. Intern. Med. 154:26–34.
Angus D. C., W. T. Linde-Zwirble, J. Lidicker, G. Clermont, J. Carcillo, and M. R. Pinsky. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29:1303–1310.
Das, U. N. 2003. Current advances in sepsis and septic shock with particular emphasis on the role of insulin. Med. Sci. Monit. 9:RA181–192.
Das U. N. 2000. Critical advances in septicemia and septic shock. Crit. Care 4:290–296.
Heidemann, S. M., L. Lomo, J. P. Ofenstein, and P. Samaik. 2000. The effect of heat on cytokine production in rat endotoxemia. Crit. Care Med. 28:1465–1468
Vincent, J. L. 1997. New therapies in sepsis. Chest 112:330–338.
Michie, H. R. 1996. Metabolism of sepsis and multiple organ failure. World J. Surg. 20:460–464.
Jakab, G. J., E. W. Spannhake, B. J. Canning, S. R. Kleeberger, and M. I. Gilmour. 1995. The effects of ozone on immune function. Environ. Health Perspect. 103(Suppl 2):77–89.
Bocci, V. 1996. Does ozone therapy normalize the cellular redox balance? Med. Hyphotheses 46:150–154.
Bocci, V. 1998. Is ozone therapy therapeutic? Perspect. Biol. Med. 42:131–143.
Bocci, V. 1999. Biological and clinical effects of ozone. Has ozone therapy a future in medicine? Br. J. Biomed. Sci. 56:270–279.
Canada, A. T., E. J. Calabrese, and D. Leonard. 1986. Age-dependent inhibition of pentobarbital sleeping time by ozone in mice and rats. J. Gerontol. 41:587–589.
Rilling, S., and R. Viebahn. 1990. Praxis der Ozone-Sauerstoff-Theorie. Ed. E. Fisher Verlag, Heidelberg.
Wielgus-Serafińska, E., A. Plewka, and M. Kamiński. 1993. Circadian variation of mitochondrial succinic dehydrogenase and microsomal cytochrome P-450 dependent monooxygenase activity in the liver of sexually immature and mature rats. J. Physiol. Pharmacol. 44:55–63.
Czekaj, P., A. Plewka, M. Kamiński, G. Nowaczyk, K. Pawlicki, and E. Wielgus-Serafińska. 1994. Daily and circadian rhythms in the activity of mixed function oxidases system in rats of different age. Biol. Rhythm. Res. 25:67–75.
Wachstein, M., and E. Meisel. 1957. Histochemistry of hepatic phosphatases at physiologic pH with special reference to the demonstration of bile canaliculi. Ann. J. Clin. Path. 27:12–23, 1957.
Gomori, G. 1953. Microscopic Histochemistry. Principles and Practice. The University of Chicago Press, pp 137–224.
Vorbrodt, A. 1964. Histochemical methods of phosphatases identification. Ed. Krygier A. Vol. 6, Warszawa, pp 135–152.
Hayashi, M., Y. Nakajima, and W. H. Fishman. 1964. The cytologic demonstration of beta-glucoronidase emploiny naphtol AS-BI glucoronidase and hexasonium pararosanilin. J. Histochem. Cytochem. 12:293–297.
Madej, P., Z. Antoszewski, and J. A. Madej. 1995. Ozonotherapy. Mater Med. Pol. 27:53–56.
Bertoni-Freddari, C., P. Fattoretti, U. Caselli, R. Paoloni, and W. Meier-Ruge. 1996. Age-dependent decrease in the activity of succinic dehydrogenase in rat CA1 pyramidal cells: a quantitative cytochemical study. Mech. Ageing Develop. 90:53–62.
Andres, D., N. Sanz, A. Zaragoza, A. M. Alvarez, and M. Cascales. 2000. Changes in antioxidant defence systems induced by cyclosporine A in cultures of hepatocytes from 2- and 12-month-old rats. Biochem. Pharmacol. 59:1091–1100.
Malarkodi, K. P., A. V. Balachandar, and P. Varalakshmi. 2003. The influence of lipoic acid on adriamycin induced nephrotoxicity in rats. Mol. Cell. Biochem. 247:15–22.
Bartels, H., S. Freimann, and K. Jungermann. 1993. Predominant periportal expression of the phosphoenolpyruvate carboxykinase gene in liver of fed and fasted mice, hamster and rats studied by in situ hybridization. Histochemistry 99:303–309.
Loffler, M., C. Becker, E. Wegerle, and G. Schuster. 1996. Catalytic enzyme histochemistry and biochemical analysis of dihydroorotata dehydrogenase/oxidase and succinate dehydrogenase in mammalian tissues, cells and mitochondria. Histochem. Cell Biol. 105:119–128.
Mansuy, D. 1998. The great diversity of reactions catalyzed by cytochromes P450. Comp. Biochem. Physiol. 121:5–14.
Plewka, A., M. Bienioszek, and D. Plewka. 1994. Changes in the male rat hepatic cytochrome P-450 level, heme oxygenase and δ-aminolevulinic acid synthase activities at various stages of life. Mech. Ageing. Develop. 74:79–88.
Strumiło, S., and J. Czerniecki. 1996. Comparison of isoenzyme composition and kinetic properties of lactate dehydrogenase from rabbit and hare hearts. Biochem. Archiv. 12:85–88.
Lee, P. Ch., B. Jelinek., M. Struve, E. D. Bruder, and H. Raff. 2000. Effect of neonatal hypoxia on the development of hepatic lipase in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279:R1341–1347.
Bagchi, D., M. Bagchi, E. A. Hassoun, and S. J. Stohs. 1995. In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology 104:129–140.
Plewka, A., M. Kamiński, and D. Plewka. 1998. Ontogenesis of hepatocyte respiration processes in relation to metabolism of xenobiotics. Mech. Ageing. Develop. 105:197–207.
Mochitate, K., K. Ishida, T. Ohsumi, and T. Miura. 1992. Long-term effects of ozone and nitrogen dioxide on the metabolism and population of alveolar macrophages. J. Toxicol. Environ. Health 35:247–260.
Mochitate, K., and T. Miura. 1989. Metabolic enhancement and increase of alveolar macrophages induced by ozone. Environ. Res. 49:79–92.
Muriel, P. 1995. Interferon-α preserves erythrocyte and hepatocyte ATPase activities from liver damage induced by prolongated bile duct ligation in the rat. J. Appl. Toxicol. 15:449–453.
Tinton, S. A., V. H. Lefebvre, O. C. Cousin, and P. M. Buc-Calderon. 1993. Cytolytic effects and biochemical changes induced by extracellular ATP to isolated hepatocytes. Biochim. Biophys. Acta. 1176:1–6.
Arivazhagan, P., K. Ramanathan, C. Panneerselvam. 2001. Effect of DL-alpha-lipoic acid on mitochondrial enzymes in aged rats. Chem. Biol. Interact. 138:189–198.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madej, P., Plewka, A., Madej, J.A. et al. Ozone Therapy in Induced Endotoxemic Shock. II. The Effect of Ozone Therapy Upon Selected Histochemical Reactions in Organs of Rats in Endotoxemic Shock. Inflammation 30, 69–86 (2007). https://doi.org/10.1007/s10753-007-9023-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-007-9023-5