Abstract
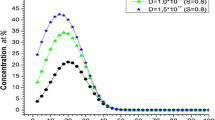
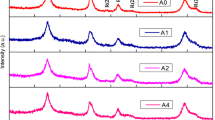
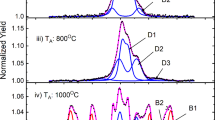
57Fe doped titanium oxide monocrystals, prepared by ion implantation at different temperatures and subsequent thermal treatment, were characterized by conversion electron Mössbauer spectrometry, synchrotron radiation x-ray diffraction and superconducting quantum interference device magnetometry. After implantation at room temperature Fe is present in divalent state. Upon annealing in high vacuum Fe2 + is reduced to metallic Fe for the most part. After implantation at 623 K most iron is in metallic state. During annealing on air Fe is gradually oxidized from Fe2 + to Fe3 + . Depending on preparation conditions and thermal treatment the role of different nanosized secondary phases is discussed in terms of their influence on the magnetic properties of Fe:TiO2. α-Fe nanoparticles are found to be responsible for ferromagnetism observed in TiO2.
Similar content being viewed by others
References
Matsumoto, Y., et al.: Science 291, 854–855 (2001)
Wolf, S.A., et al.: Science 294, 1488–1495 (2001)
Kim, K.J., et al.: J. Appl. Phys. 99, 08M120-1 (2006)
Hong, N.H., et al.: Phys. Rev. B 70, 195204-1 (2004)
Sangaletti, L., et al.: J. Phys.: Condens. Matter 18, 7643–7650 (2006)
Shutthanandan, V., et al.: Appl. Phys. Lett. 84, 4466–4468 (2004)
Xu, J.P., et al.: Solid State Commun. 140, 514–518 (2006)
Osterwalder, J., et al.: Thin Solid Films 484, 289–298 (2005)
Zhang, X., et al.: J. Phys. D: Appl. Phys. 41, 1–5 (2008)
Xin, Y., et al.: Appl. Phys. Lett. 88, 112512-1 (2006)
Kim, Y.J., et al.: Appl. Phys. Lett. 84, 3531–3533 (2004)
Nomura, K., et al.: Thin Solid Films 515, 86498652 (2007)
Nomura, K., et al.: Hyperfine Interact. 168, 1065–1071 (2006)
Dietl, T.: J. Appl. Phys. 103, 07D111-4 (2008)
Rodríguez Torres, C.E., et al.: Physica B 354, 67–70 (2004)
Zhu, S., et al.: Physica B 364, 199–205 (2005)
Rodríguez Torres, C.E., et al.: J. Phys., Condens. Matter 20, 135210 (2008)
Balcells, Ll., et al.: Appl. Phys. Lett. 89, 122501-1 (2006)
Hong, N.H., et al.: Phys. Rev., B 73, 132404-1 (2006)
Potzger, K., et al.: Appl. Phys. Lett. 92, 182504 (2008)
Zhou, S., et al.: J. Appl. Phys. 103, 07D530 (2008)
Brand, R.A.: Nucl. Instrum. Methods B 28, 398 (1987)
Long, G.J.: Mössbauer Spectroscopy Applied to Inorganic Chemistry, p. 507. Plenum, Pittsburg (1987)
Shirane, G., et al.: Phys. Rev. 125, 1158 (1962)
Guermazi, M., et al.: Mat. Res. Bull. 18, 529–538 (1983)
Zhou, S., et al.: J. Appl. Phys. 103, 083907 (2008)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Talut, G., Reuther, H., Grenzer, J. et al. Origin of ferromagnetism in iron implanted rutile single crystals. Hyperfine Interact 191, 95–102 (2009). https://doi.org/10.1007/s10751-009-9958-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10751-009-9958-z