Abstract
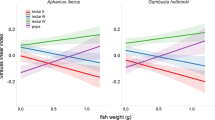
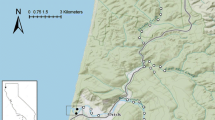
The mosquito Aedes phoeniciae is a potential disease vector that inhabits the coastal rock-pools of the Southeastern Mediterranean Sea. Our year-long study examined the abundance and distribution of Ae. phoeniciae in 49 rock-pools along HaBonim Beach Nature Reserve (Israeli coast) on a monthly basis (September 2016 to August 2017). Additionally, the physical, chemical, and biological characteristics of the rock-pools were measured. Our results showed a correlation between the abundance of Ae. phoeniciae and abiotic (salinity, pool volume, and pH) and biotic (bacterial, micro-phytoplankton, and chironomid abundance) characteristics. A complementary experiment was conducted to examine the role of bacteria and phytoplankton on Ae. phoeniciae larval performance by rearing larvae in seawater (SW) or seawater without microbes (FSW, 0.2-µm). Ae. phoeniciae grown in SW exhibited a high survivorship rate (~ 77%), while lower survivorship rate was measured in the FSW treatments (~ 45%). Furthermore, a higher number of adult females were found in the SW compared to FSW treatments (35 and 11, respectively), while the number of male adults remained similar. Our results suggest that Ae. phoeniciae larvae rely on the water characteristics and especially on the microbial communities that habitat the rock-pools. These results may enable improved mosquito control of Ae. phoeniciae along the Southeastern Mediterranean Sea.





Similar content being viewed by others
References
Becker, N., D. Petric, M. Zgomba, C. Boase, M. Madon, C. Dahl & A. Kaiser, 2010. Mosquitoes and their control, Vol. 577. Springer, Heidelberg. https://doi.org/10.1007/978-3-540-9287-4.
Bedhomme, S., P. Agnew, C. Sidobre & Y. Michalakis, 2003. Sex-specific reaction norms to intraspecific larval competition in the mosquito Aedes aegypti. Journal of Evolutionary Biology 16: 721–730.
Blaustein, L. & J. M. Chase, 2007. Interactions between mosquito larvae and species that share the same trophic level. Annual Review of Entomology 52: 489–507.
Blaustein, L. & B. P. Kotler, 1993. Oviposition habitat selection by the mosquito, Culiseta longiareolata: effects of conspecifics, food and green toad tadpoles. Ecological Entomology 18: 104–108.
Blaustein, L. & S. S. Schwartz, 2001. Why study ecology in temporary pools? Israel Journal of Zoology 47: 303–312.
Blaustein, L., M. Kiflawi, A. Eitam, M. Mangel & J. E. Cohen, 2004. Oviposition habitat selection in response to risk of predation in temporary pools: mode of detection and consistency across experimental venue. Oecologia 138: 300–305.
Brendonck, L., M. Jocqué, K. Tuytens, B. V. Timms & B. Vanschoenwinkel, 2015. Hydrological stability drives both local and regional diversity patterns in rock pool metacommunities. Oikos 124: 741–749.
Campbell-Lendrum, D., L. Manga, M. Bagayoko & J. Sommerfeld, 2015. Climate change and vector-borne diseases: what are the implications for public health research and policy? Philosophical Transactions of the Royal Society B-Biological Sciences 370: 1–8.
Cheng, L., 1976. Marine Insects. North-Holland Publishing Company, Amsterdam: 581.
Clements, A. N., 1999. The Biology of Mosquitoes: Sensory, Reception and Behaviour, Vol. 2. CABI, Wallingford.
Coluzzi, M. & A. Sabatini, 1968. Morphological divergences and sterility barriers in the Aedes mariae complex (diptera, culicidae). Rivista Di Parassitologia 29: 49–70.
Coluzzi, M., L. Bullini & A. P. Bianchi Bullini, 1971. Phosphoglucomutase polymorphism in Aedes phoeniciae Coluzzi & Sabatini of the Ae. mariae complex (Dipt., Culicidae). Bulletin of Entomological Research 61: 327–331.
Coumou, D. & S. Rahmstorf, 2012. A decade of weather extremes. Nature Climate Change 2: 491–496.
Dieng, H., C. Mwandawiro, M. Boots, R. Morales, T. Satho, N. Tuno, Y. Tsuda & M. Takagi, 2001. Leaf litter decay process and the growth performance of Aedes albopictus larvae (Diptera: Culicidae). Journal of Vector Ecology 27: 31–38.
Duguma, D., M. G. Kaufman & A. B. Simas Domingos, 2017. Aquatic microfauna alter larval food resources and effect development and biomass of West Nile and Saint Louis encephalitis vector Culex nigripalpus (Diptera: Culicidae). Ecology and Evolution 7: 3507–3519.
Eitam, A., L. Blaustein & M. Mangel, 2002. Effects of Anisops sardea (Hemiptera: Notonectidae) on oviposition habitat selection by mosquitoes and other dipterans and on community structure in artificial pools. Hydrobiologia 485: 183–189.
Goldreich, Y., 1995. Temporal variations of rainfall in Israel. Climate Research 5: 167–179.
Hassaine, K., S. Gourmala & G. Metge, 2001. Cinétique démographique des populations pré-imaginales d’Aedes mariae (Diptera: Culicidae) des côtes occidentales algériennes. Annales de Limnologie 37: 59–69.
Herbst, D. B., 2001. Gradients of salinity stress, environmental stability and water chemistry as a templet for defining habitat types and physiological strategies in inland salt waters. Hydrobiologia 466: 209–219.
Herms, W. B., 1928. The effect of different quantities of food during the larval period on the sex ratio and size of Lucilia sericata meigen and Theobaldia incidens (Thom.). Journal of Economic Entomology 21: 720–729.
Hidalgo, K., J. P. Dujardin, K. Mouline, R. K. Dabiré, D. Renault & F. Simard, 2015. Seasonal variation in wing size and shape between geographic populations of the malaria vector, Anopheles coluzzii in Burkina Faso (West Africa). Acta Tropica 143: 79–88.
Honório, N. A., P. H. Cabello, C. T. Codeço & R. Lourenço-de-oliveira, 2006. Preliminary data on the performance of Aedes aegypti and Aedes albopictus immatures developing in water-filled tires in Rio de Janeiro. Memórias do Instituto Oswaldo Cruz 101: 225–228.
Imam, H., G. S. Zarnigar & S. Aziz, 2014. The basic rules and methods of mosquito rearing (Aedes aegypti). Tropical Parasitology 4: 53–56.
Jocque, M., B. Vanschoenwinkel & L. Brendonck, 2010. Freshwater rock pools: a review of habitat characteristics, faunal diversity and conservation value. Freshwater Biology 55: 1587–1602.
Juliano, S. A., 2009. Species interactions among larval mosquitoes: context dependence across habitat gradients. Annual Review of Entomology 54: 37–56.
Kauffman, E., A. Payne, M. A. Franke, M. A. Schmid, E. Harris & L. D. Kramer, 2017. Rearing of Culex spp. and Aedes spp. mosquitoes. Bio-Protocol 7(17): 1–25.
Khasnis, A. A. & M. D. Nettleman, 2005. Global warming and infectious disease. Archives of Medical Research 36: 689–696.
Kneitel, J. M., 2007. Intermediate-consumer identity and resources alter a food web with omnivory. Journal of Animal Ecology 76: 651–659.
Kolasa, J. & T. N. Romanuk, 2005. Assembly of unequal world of a rock pool metacommunity. In Holyoak, M., M. A. Leobold & R. D. Holt (eds), Metacommunities: Spatial Dynamics and Ecological Communities. University of Chicago Press, Chicago: 212–232.
Kress, N. & B. Herut, 2001. Spatial and seasonal evolution of dissolved oxygen and nutrients in the Southern Levantine Basin (Eastern Mediterranean Sea): chemical characterization of the water masses and inferences on the N:P ratios. Deep-Sea Research Part I: Oceanographic Research Papers 48: 2347–2372.
Langenheder, S. & H. Ragnarsson, 2007. The role of environmental and spatial factors for the composition of aquatic bacterial communities. Ecology 88: 2154–2161.
Lensky, N. G., Y. Dvorkin, V. Lyakhovsky, I. Gertman & I. Gavrieli, 2005. Water, salt, and energy balances of the Dead Sea. Water Resources Research 41: 1–13.
Lipkin, Y. & U. Safriel, 1971. Intertidal zonation on rocky shores at Mikhmoret (Mediterranean, Israel). The Journal of Ecology 59: 1–30.
Mastrantonio, V., D. Porretta, R. Bellini, G. Nascetti & S. Urbanelli, 2015. Molecular systematics and origin of the Mediterranean sea rock-pool mosquitoes of the Aedes mariae (Diptera: Culicidae) Complex. Annuals of the Entomological Society of America 108: 593–599.
McGregor, D. D., 1965. Physical ecology of some New Zealand supralittoral pools. Hydrobiologia 25: 277–284.
Naeem, S., 1988. Oecologia Predator-prey interactions and community structure: chironomids, mosquitoes and copepods. Oecologia 77: 202–209.
Ozer, T., I. Gertman, N. Kress, J. Silverman & B. Herut, 2016. Interannual thermohaline (1979–2014) and nutrient (2002–2014) dynamics in the Levantine surface and intermediate water masses, SE Mediterranean Sea. Global Planetary Change 151: 60–67.
Porretta, D., D. Canestrelli, R. Bellini, G. Celli & S. Urbanelli, 2007. Improving insect pest management through population genetic data: a case study of the mosquito Ochlerotatus caspius (Pallas). Journal of Applied Ecology 44: 682–691.
Quinn, G. P. & M. J. Keough, 2002. Experimental design and Data Analysis for Biologists. Cambridge University Press, Cambridge. https://doi.org/10.15713/ins.mmj.3.
Rahav, E., A. Paytan, E. Mescioglu, Y. Galletti, S. Rosenfeld, O. Raveh, C. Santinelli, T. Ho & B. Herut, 2018. Airborne microbes contribute to N2 fixation in surface water of the Northern Red Sea. Geophysical Research Letters. https://doi.org/10.1029/2018GL077132.
Raveh, O., N. David, G. Rilov & E. Rahav, 2015. The temporal dynamics of coastal phytoplankton and bacterioplankton in the eastern mediterranean sea. PLoS One 10: 1–23.
Reiskind, M. H., K. L. Greene & L. P. Lounibos, 2009. Leaf species identity and combination affect performance and oviposition choice of two container mosquito species. Ecological Entomology 34: 447–456.
Reitz, S. R. & J. T. Trumble, 2002. Competitive displacment among insects and arachnids. Annuals of the Entomological Society of America 47: 435–465.
Rilov, G., 2016. Multi-species collapses at the warm edge of a warming sea. Scientific Reports 6(36897): 1–14.
Rioux, J. A., 1958. Les Culicidés du midi Méditerranéen. Encyclopedie Entomologiqu XXXV.
Rosenfeld, S., D. Porretta, E. Rahav, V. Mastrantonio, C. Duchet & L. Blaustein, 2018. Scientific Note Molecular identification of Aedes phoeniciae (Diptera: Culicidae) in rockpools along the northern Israeli coast. Journal of Vector Ecology 43: 1–3.
Schönfeld, J., E. Alve, E. Geslin, F. Jorissen, S. Korsun, S. Spezzaferri, S. Abramovich, A. Almogi-Labin, E. A. du Chatelet, C. Barras, L. Bergamin, E. Bicchi, V. Bouchet, A. Cearreta, L. Di Bella, N. Dijkstra, S. T. Disaro, L. Ferraro, F. Frontalini, G. Gennari, E. Golikova, K. Haynert, S. Hess, K. Husum, V. Martins, M. McGann, S. Oron, E. Romano, S. M. Sousa & A. Tsujimoto, 2012. The FOBIMO (FOraminiferal BIo-MOnitoring) initiative-towards a standardised protocol for soft-bottom benthic foraminiferal monitoring studies. Marine Micropaleontology 94–95: 1–13.
Sergent, E. & E. Sergent, 1903. Observations sur les moustiques des environs d’Alger. Annales de I’Institut Pasteur 17: 60–67.
Silberbush, A., I. Tsurim, Y. Margalith & L. Blaustein, 2014. Interactive effects of salinity and a predator on mosquito oviposition and larval performance. Oecologia 175: 565–575.
Silver, J. B., 2008. Mosquito Ecology: Field Sampling Methods, 2nd ed. Springer Science & Business Media, Dordrecht.
Sisma-Ventura, G., R. Yam, N. Kress & A. Shemesh, 2016. Water column distribution of stable isotopes and carbonate properties in the South-eastern Levantine basin (Eastern Mediterranean): vertical and temporal change. Journal of Marine Systems 158: 13–25.
Spencer, M., L. Blaustein & J. E. Cohen, 2002. Oviposition habitat selection by mosquitoes (Culiseta longiareolata) and consequences for population size. Ecology 83: 669–679.
Sushanth, V. R. & M. Rajashekhar, 2015. Effect of twelve species of marine phytoplankton on larval survival and development of the mosquito Culex quinquefasciatus. International Journal of Marine Science 5: 1–5.
Theobald, F. V., 1907. A Monograph of the Culicidae, or Mosquitoes, Vol. 4. Longmans & Co, Harlow.
Therriault, T. W. & J. Kolasa, 2001. Desiccation frequency reduces species diversity and predictability of community structure in coastal rock pools. Israel Journal of Zoology 47: 477–489.
Trexler, J. D., C. S. Apperson, L. Zurek, C. Gemeno, C. Schal, M. Kaufman, E. Walker, D. W. Watson & L. Wallace, 2003. Role of bacteria in mediating the oviposition responses of Aedes albopictus (Diptera: Culicidae). Journal of Medical Entomology 40: 841–848.
Urbanelli, S., D. Porretta, V. Mastrantonio, R. Bellini, G. Pieraccini, R. Romoli, G. Crasta & G. Nascetti, 2014. Hybridization, natural selection, and evolution of reproductive isolation: a 25-years survey of an artificial sympatric area between two mosquito sibling species of the Aedes mariae complex. Evolution 68: 3030–3038.
Wallace, J. B. & W. R. Merritt, 1980. Filter-feeding ecology of aquatic insects. Annual Reviews of Entomology 25: 103–132.
Wei, P., 2001. Akaike’s information criterion in generalized estimating equations. Biometrics 57: 120–125.
Welschmeyer, N. A., 1994. Fluorometric analysis of chlorophyll-a in the presence of chlorophyll b and pheo-pigments. Limnology and Oceanography 39: 1985–1992.
Wilkerson, R. C., Y. M. Linton, D. M. Fonseca, T. R. Schultz, D. C. Price & D. A. Strickman, 2015. Making mosquito taxonomy useful: a stable classification of tribe Aedini that balances utility with current knowledge of evolutionary relationships. PLoS One 10: 1–26.
Yavasoglu, S. I., F. M. Simsek & C. Ulger, 2016a. Distribution pattern and genetic structure of Aedes zammitii (Diptera: Culicidae) along the Mediterranean and Aegean coasts of Turkey. Journal of Vector Ecology 41: 151–159.
Yavasoglu, S. I., C. Yilmaz, C. Ulger & F. M. Simsek, 2016b. Molecular identification and genetic structure of Aedes phoeniciae (Diptera: Culicidae) in Northern Cyprus and Turkey. Biochemical Systmatics and Ecology 69: 6–14.
Yee, D. A. & S. A. Juliano, 2006. Consequences of detritus type in an aquatic microsystem: effects on water quality, micro-organisms and performance of the dominant consumer. Freshwater Biology 51: 448–459.
Acknowledgements
We are most grateful to Valentina Rovelli, Nadav Pezaro, Luca Zoccarato, Martina Mulas, and Alvaro Israel for their help. This work was supported by an Israel Science Foundation Grant 891/2012 awarded to LB and by a Ministry of Environmental Protection Grant (Number 145–1–2) awarded to ER. JK was supported by the Fulbright Scholar Program and the United States-Israel Educational Foundation. This study is in partial fulfillment of an MSc thesis of Sahar Rosenfeld from the University of Haifa.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Iacopo Bertocci
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rosenfeld, S., Blaustein, L., Kneitel, J. et al. The abundance and larval performance of Aedes phoeniciae in supralittoral rock-pools. Hydrobiologia 846, 181–192 (2019). https://doi.org/10.1007/s10750-019-04063-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-04063-6