Abstract
Detailed knowledge on the habitat preference of invasive fishes and the bias of different fishing methods in determining their population dynamic parameters are essential in fisheries management, ecology and conservation. This study was conducted to determine the habitat use and length frequency distribution of the invasive monkey goby and pumpkinseed in the littoral zone of Lake Balaton (Hungary) using two different sampling methods, electrofishing and fyke netting. In general, both species preferred anthropogenically modified habitat types (rip-rap shorelines and harbours) compared with natural reed habitats with silty-sand bottom. Length frequency distribution data showed significant between-gear differences, since electrofishing resulted in the capture of larger individuals in greater proportion than fyke nets for both species. This study, which includes the first detailed data about the habitat use of the highly invasive monkey goby in lakes, suggests that invasive species may benefit from the alteration of the littoral zone. It also highlights that reliance on single gear surveys can be misleading in assessing habitat use and population structure of invasive fishes.
Similar content being viewed by others
Introduction
Biological invasions are major contributors to the loss of biodiversity and the homogenisation of biota worldwide (Rahel, 2000; Clavero & García-Berthou, 2005; Dudgeon et al., 2006). Human activity is rapidly transforming natural ecosystems and habitats which alterations often facilitate the spreading of non-native species (Byers, 2002; Johnson et al., 2008). Fishes are amongst the most common introduced taxa worldwide, mainly for societal demands of fish products, angling and ornamental market (Gozlan, 2008). However, introduction of non-native freshwater fishes can often cause catastrophic ecological consequences (Vitule et al., 2009). Lakes are particularly vulnerable to introduction of non-native fishes and these ecosystems often maintain several unique endemism. For instance, the introduction of Nile perch [Lates niloticus (Linnaeus, 1758)] along with several tilapiine species into Lake Victoria caused dramatic reductions of the previously abundant endemic haplochromine species (Hughes, 1986; Ogutu-Ohwayo, 1990; Witte et al., 1992). For predicting the ecosystem impacts of non-native species and developing more effective management strategies to control their negative impacts and spreading, detailed information about their spatial distribution and habitat preference is a prerequisite (Cooper et al., 2009; Verhelst et al., 2015).
Identifying effective sampling techniques of non-native fishes is crucial to estimate their abundances, and also for feasible population control measures (Trebitz et al., 2009; Collins et al., 2015). It is known that different sampling gears can provide different estimations of the abundance of a given species even in the same location, which complicates the choice of an appropriate sampling method (Johnson et al., 2005; Brandner et al., 2013). Moreover, fish size selectivity and sampling cost and effort could also differ between sampling methods (e.g. Erős et al., 2009; Ribeiro et al., 2015). Although electrofishing is the most commonly used method in the routine monitoring of fish assemblages in the littoral zone of lakes (Minns et al., 1994; Pierce et al., 2001; CEN, 2003; Eggleton et al., 2010), several studies emphasise the use of complementary sampling methods for a more complete estimation of fish assemblage structure (e.g. Weaver & Magnuson, 1993; Clark et al., 2007; Bonvechio et al., 2014; Hinlo et al., 2017). Unlike other gears, fyke nets may be universally applicable for monitoring fish communities in a wide variety of littoral meso-habitats. In fact, several studies indicated that fyke nets were highly effective in catching small-bodied fishes and tended to collect more individuals and more species of fishes than other gears (Weaver & Magnuson, 1993; Clark et al., 2007; Eggleton et al., 2010). Nevertheless, less research has been devoted to compare the habitat use and population dynamic parameters of non-native fish species using different sampling methods (but see, e.g. Trebitz et al., 2009; Bauer-Haáz et al., 2014).
Several non-native fishes settled in European lakes, which may threaten biodiversity and ecosystem functioning (e.g. Musil et al., 2010; Ciutti et al., 2011; Ferincz et al., 2016; Takács et al., 2017). Yet data are sporadic about their abundance and habitat-use patterns, which may hinder the determination of population trends and eradication strategies. In the littoral zone of Lake Balaton, which is the largest shallow lake in Central Europe, the monkey goby [Neogobius fluviatilis (Pallas, 1814)] and the pumpkinseed [Lepomis gibbosus (Linnaeus, 1758)] are the most dominant invasive species (Specziár, 2010). However, compared to watercourses, few detailed data exist about their abundance and habitat-use patterns in this and other European lakes (e.g. Neophitous & Giapis 1994; Vila-Gispert & Moreno-Amich, 1998; Dembski et al., 2008; Didenko, 2013).
The monkey goby is a small, invasive benthic fish, from the Ponto–Caspian region, which was first documented in Lake Balaton in 1970 (Bíró, 1971). Previous studies suggested that monkey goby prefers inshore habitats with finer substrata (i.e. silty sand, sand, small gravel) in rivers (Erős et al., 2005; Adámek et al., 2007; Čápová et al., 2008; Borcherding et al., 2013). The habitat use of the species is understudied in lakes, although Didenko (2013) found most of the adult and sub-adult individuals on sandy bottoms. The pumpkinseed is a centrarchid fish (sunfish) native to North America. In several European countries (including Hungary), it has become widely established and form invasive populations in many, but not all cases (Cucherousset et al., 2009; Copp et al., 2017). The species inhabits both lentic and lotic environments (Robinson et al., 2000; Fox & Copp, 2014) and has various negative impacts on native fauna (Tomeček et al., 2007; Van Kleef et al., 2008). According to Bíró (1997), pumpkinseed began to colonise Lake Balaton presumably from a nearby fishpond between 1904 and 1908. Nowadays, it has stable and self-sustaining populations in the whole lake and its drainage (Bíró et al., 2003). Although these two fish species may seriously impact food web in the littoral zone of the lake via the competition of food resources and space with native fishes (Specziár, 2010), no study to date examined their habitat-use patterns, especially with different sampling methods, which may halt effective management measures.
This study was conducted to determine the habitat use and length frequency distribution of the monkey goby and the pumpkinseed in the littoral zone of Lake Balaton using two different sampling methods, electrofishing and fyke netting. We hypothesised that both non-native species might prefer anthropogenically modified habitat types (rip-rap shorelines and harbours) to natural reed covered areas with silty-sand bottom. We were especially interested to compare whether the two different sampling methods characterise the habitat-use patterns of the two species consistently. Finally, regarding the length frequency distribution of the species, we predicted that fyke netting would catch smaller individuals, and electrofishing would catch larger individuals, and hereby complement each other on the size-structured habitat use of the two species (see e.g. Chick et al., 1999; Ruetz et al., 2007; Warry et al., 2013; Francis et al., 2014 for other small-bodied species).
Materials and methods
Study area
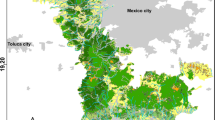
Lake Balaton (Hungary) is the largest lake (surface area: 593 km2; mean depth: 3.2 m) in Central Europe (Fig. 1). The lake is mesotrophic (Istvánovics et al., 2007). About 40% of the littoral zone is covered by common reed [Phragmites australis (Cav.) Trin. ex Steud.] (Specziár et al., 2013), but the rest of the shoreline is anthropogenically modified (mainly rip-rap sections, beaches, and harbours). Lake Balaton can be divided into four basins (Fig. 1) on the basis of large-scale circulation patterns (Istvánovics et al., 2007). Detailed information on the limnology of the lake can be found in Istvánovics et al. (2007).
Fish sampling
We sampled fishes during three sampling periods in 2017: (1) spring (from 24 April to 04 May), summer (from 12 July to 19 July), and autumn (from 18 September to 11 October). Three distinct mesohabitat types can be distinguished in the littoral zone of the lake: (1) semi-natural reed covered areas, (2) rip-rap shoreline and (3) harbours. Reed habitats had a depth of 40–140 cm and had silty-sand bottom. Reed strip width along the shoreline varied between 8 and 40 m. The rip-rap shorelines consisted of large rocks and boulders with diameter size of 20–40 cm. They had a depth of 35–140 cm and they gradually sloped. The harbours could be characterised by deep water (110–330 cm) and heavy boat traffic. Most part of the shoreline of this habitat type was strengthened by concrete which was usually perpendicular to the bottom creating a concrete “wall”. Substrate of the harbours consisted of silty sand. Electrofishing and fyke netting were performed in reed and rip-rap habitats, but only fyke nets were used in harbours due to deep water and the high number of ships and sailboats, which would not have allowed electrofishing. Two reed habitats, two rip-rap habitats and two harbours were sampled in each of the four basins of Lake Balaton (Fig. 1). This sampling effort yielded a total of 120 samples (for electrofishing: three seasons × four basins × four sampling sites, and for fyke nets: three season × four basins × six sampling sites).
Electrofishing was performed from a rubber boat during daytime following European standard protocols (CEN, 2003) using backpack electrofishing gear (IG200/2B, PDC, 50–100 Hz, 350–650 V, max. 10 kW; Hans Grassl GmbH, Germany). Pulsating direct current with a frequency of 75–90 Hz and a voltage of 200–300 V was used. Mean total length of the sampled sections was 156 m (± 45 m SD). Reed habitats were sampled by driving the boat directly along the reed strip and open water border and also between the reed tufts by moving the boat into the reeds. Gradually sloped rip-raps allowed the boat to sail above this habitat type and thereby to sample the rip-rap zone directly by electrofishing. The crew comprised of two persons: one for catching the fish with the hand-held anode (2.5-m-long pole with a net of 40 cm diameter, mesh size 6 mm) and one for driving the boat. The cathode, a 5-m-long copper cable, was floated at the rear of the boat. Anesthetised fish were netted directly with the anode by the operator then placed into a 100-L, water-filled tank in the boat. Standard length of most fish was measured to the nearest 1 mm. Monkey goby and pumpkinseed were euthanised with clove oil and placed on ice. Individuals of other species were released immediately after measurement.
Fyke netting was performed in the same habitats as electrofishing and in two harbours per basin from a boat (Fig. 1). The net frame has a length of 90 cm divided into a series of 50-, 45- and 40-cm diameter hoops, and it has a single 130-cm-long wing and an easily expanding 15 cm throat size. Mesh size of the net was 6 mm. Deployment and retrieval of fyke nets occurred in the afternoon and in the following morning, respectively. The mean sampling time period (hours between deployment and retrieval) of the nets was 16.3 ± 1.7 h (± SD). Five fyke nets were set at each sampling site along a 150-m shoreline with equal distances (around 25 m) from each other, which resulted in the deployment of 120 fyke nets per season. Nets were set perpendicular to the shoreline as close as possible to shore. The nets had two steel rods and one plastic tube fastened lengthwise to their lower and upper parts, respectively. Each fyke net was marked by a buoy and identified by a GPS device (Garmin GPSMAP 64, https://www.garmin.com/en-US/). Similar to electrofishing, individuals of monkey goby and pumpkinseed were euthanised and placed on ice. Water depth and Secchi depth were also measured on each sampling occasion at each sampling site.
Statistical analysis
As a methodological aspect, linear regression was used to test the effect of sampling time period of fyke netting (hours between deployment and retrieval) on the abundance of both species (the effect of sampling time period was not significant: for monkey goby: adj. R2 = 0.02, P = 0.134 and for pumpkinseed: adj. R2 = 0.008, P = 0.502).
Generalized additive models (GAM) using identity link functions with Gaussian error distribution were used to determine the effects of the explanatory variables on abundance of monkey goby and pumpkinseed. GAM is an extension of the generalized linear model (GLM) which determines the shape of the response curves from the data (i.e. smooth function), instead of fitting an a priori parametric model (Hastie & Tibshirani, 1986; Wood, 2006). For each smooth function, a maximum number of three degrees of freedom (k = 4) were used. Season, habitat and their interaction as categorical factors and sampling depth and Secchi depth as continuous variables were used in the models as explanatory variables. Secchi depth was expressed as the proportion of transparency of the water column (i.e. Secchi depth/sampling depth ratio) and transformed by arcsin-square root prior to statistical analysis. Altogether, four separate models were run (two species × two gears). CPUE data (number of individuals/sample length (m)) and cumulative abundance data (five fyke nets/site) of the two species were used as response variables for analysing data collected by electrofishing and fyke nets, respectively. Prior to analysis, fourth-root transformation was performed on response variables to approach homoscedasticity and meet the assumption of normality (Wood, 2006). A backward stepwise procedure was used to select the final models, whereby we eliminated non-significant model terms until we reached the most parsimonious models. Single models were compared by ANOVA (Crawley, 2005).
Between-gear differences in length distribution separately for pumpkinseed and monkey goby were compared using Chi-square test for independent samples. Prior to calculations, some size categories were pooled to avoid expected values less than 5. For monkey goby, < 30 mm and ≥ 90 mm length individuals were pooled into a small and a big size group, respectively (number of size groups: 7, interval: 10 mm). For pumpkinseed, only individuals ≥ 120 mm length were pooled into a big size group (number of size groups: 11, interval: 10 mm).
An alpha value of 0.05 was used to determine statistical significance of all tests. All data analyses were performed in the R statistical environment (R Core Team, 2015). GAM was conducted with the R package named “mgcv” (Wood, 2006). The “loess” function was used to fit a local regression model with depth as a continuous explanatory variable and CPUE data as response variable. This function uses non-parametric techniques to produce a smoothed model surface (Cleveland et al., 1992, Crawley, 2005). R and Microsoft Excel programs were used to create the figures.
Results
A total of 12,397 individuals representing 26 species were collected during the study (Table 1). Pumpkinseed and monkey goby were the third (1506 ind.) and fifth (888 ind.) most abundant fish species caught, respectively.
For the GAMs, the interaction term of season and habitat was excluded from three of the four final models, according to model comparisons by ANOVA, as they were not significantly worse (monkey goby-electrofishing: P = 0.162, monkey goby-fyke netting: P = 0.802, pumpkinseed-electrofishing: P = 0.672) than more complex models, which included interaction terms. The season and habitat interaction term has been retained only in the final model of pumpkinseed sampled by fyke nets, as model simplification did not justify its omission (P = 0.018). For electrofishing samples, habitat type and depth significantly affected the abundance both of monkey goby and pumpkinseed (Table 2). Both species were significantly more abundant in rip-rap habitats than in reed habitats (Fig. 2a). Monkey goby abundance decreased with increasing depth, while pumpkinseed abundance was the highest at medium depths (Fig. 2a). For fyke net samples, monkey goby abundance was not significantly determined by any of the explanatory variables included in the model (Table 2). In contrast, habitat and season × habitat interaction were the significant variables determining pumpkinseed abundance (Table 2). Most individuals were caught in harbours (Fig. 2b).
Number of individuals of monkey goby and pumpkinseed caught by electrofishing (standardised for 150-m sample length) (a) and fyke netting (b) by habitat types, and in relation to water depth. Thick lines: median; Boxes: upper and lower quartiles; Whiskers: minimum and maximum excluding outliers; Dots: outliers. Extreme outliers were marked with notes and their values. The “loess” function was used to draw curves on scatterplots (see “Materials and Methods” section). Response curves were added to those scatterplots only, where the relationship was significant. Note that scales on the y axes are different between rows
The length frequency distributions of the two species showed significant differences (Chi-square test) between electrofishing and fyke netting for monkey goby (χ2 = 10.49, df = 6, P = 0.033) and for pumpkinseed (χ2 = 222.58, df = 10, P < 0.001) (Fig. 3). Electrofishing caught larger-bodied individuals in greater proportion than fyke nets. Changes in catch efficiency between the two gear types happened at about 60–70-mm length in both species. Below these length classes fyke nets caught individuals in greater proportions than electrofishing (Fig. 3).
Discussion
In this study, we investigated the habitat preference of two invasive fish species with two sampling methods in a large shallow lake in Central Europe. We found increased use of anthropogenic habitats compared with natural reed habitat for both species. Human-induced habitat modification is known to facilitate fish invasions (Wiesner, 2005; Light & Marchetti, 2007; Clavero et al., 2013). Since the 1970s, extent of the natural reed area has been continuously decreasing in Lake Balaton, mainly at places where harbours and beaches had been built (Kovács et al., 1989; Zlinszky, 2013). As nearly 60% of the littoral zone of the lake now functions as harbours or strengthened by rip-rap or concrete linings against erosion, both species can find suitable habitats for maintaining stable populations. It is likely that they will further benefit from the expansion of these habitat types in Lake Balaton.
Our results about monkey goby habitat use in Lake Balaton were inconsistent with earlier findings in literature. Several studies found that the species prefers fine (i.e. silty sand, sand) substrata (Erős et al., 2005; Adámek et al., 2007; Čápová et al., 2008; Borcherding et al., 2013). In contrast, we caught significantly more individuals in rip-raps than in reed habitats with silty-sand bottom by electrofishing. Although mean monkey goby abundance was higher in reed habitats than in other habitat types sampled by fyke netting, this difference was not significant. We believe the most plausible explanation for the higher abundance of the monkey goby in rip-raps is the low rate of competition for rip-rap habitats. In Lake Balaton, only the tubenose goby [Proterorhinus marmoratus (Pallas, 1814)] can be considered to compete for spaces between rocks and boulders with monkey goby amongst fishes of the lake. In contrast, in several European rivers (e.g. Danube, Rhine, Vistula), monkey goby coexist with some other, more aggressive gobiid species (Erős et al., 2008; Grabowska et al., 2008; Roche et al., 2013; Janáč et al., 2018). As these counterparts prefer rocky shores [round goby Neogobius melanostomus (Pallas, 1814): e.g. Erős et al., 2005; Johnson et al., 2005; Kornis et al., 2012; bighead goby Ponticola kessleri (Günther, 1861): e.g. Erős et al., 2008; Borcherding et al., 2013], they may be able to outcompete monkey goby on rip-rap shoreline and force it to suboptimal habitats. In fact, interstices between large rocks and boulders may provide a more appropriate refuge from potential predators (both fish and birds) for the monkey goby than reed habitats with sandy-silt substrata where finding shelters is more difficult.
According to GAM, depth significantly affected monkey goby abundance using electrofishing samples. The number of individuals caught decreased with increasing depth from ca. 1 m. This trend can be explained by the preference for shallow water by monkey goby (Borcherding et al., 2013). However, in fyke net samples, depth was not a significant explanatory variable suggesting that this pattern more likely resulted from sampling bias of electrofishing, since increasing depth encumbers the observation of anesthetised fish (Cowx, 1996). These results suggest that monkey goby does not have clear preference for water depth in the shallow Lake Balaton.
We found that pumpkinseed showed stronger preference towards anthropogenically modified habitats in Lake Balaton than monkey goby, since most individuals were caught in rip-rap habitats by electrofishing and in rip-rap habitats and harbours by fyke netting. However, significant interaction was found between seasons and habitats in fyke net samples, which makes the significant habitat effect difficult to explain. Moreover, electrofishing samples did not confirm this result. In spring, we caught more individuals in reed habitats than in summer by fyke netting, while in harbours and in rip-rap habitats this pattern was the opposite. While electrofishing is not effective in deeper areas, fyke nets might have been able to catch the spawning individuals in reed habitats with silty-sand bottom in spring, which is an appropriate ground for building spawning nests for pumpkinseeds (Ingram & Odum, 1941; Danylchuk & Fox, 1996). Nevertheless, our results clearly show that most of the pumpkinseed individuals were caught in anthropogenic habitats either by electrofishing or fyke netting. Rip-rap habitats may offer optimal feeding ground for pumpkinseed, and especially for larger individuals (> 60–80 mm) that prey upon invertebrates (mainly molluscs) which are more abundant in the rocky shoreline (Mittelbach et al., 1992; Rezsu & Specziár, 2006). It is also likely that pumpkinseed prefers still habitats which are not exposed to waves (Specziár, 2010), and in this regard harbours provide ideal, wave-free habitats. Moreover, dense macrovegetation cover often emerges in harbours, which is favoured by this fish (Tomeček et al., 2007; Specziár, 2010).
In electrofishing samples, depth was a significant explanatory variable in modelling pumpkinseed abundance. Most individuals were caught at medium depths. Avoidance of shallower waters by the species can be explained by increasing predation risk. While monkey goby can hide effectively between rocks and boulders even in shallower water depth (< 60 cm), the larger mean size of pumpkinseed does not allow the species to find shelter under such conditions, exposing itself to predation, mainly by birds. As was found with monkey goby, decreasing abundance of pumpkinseed was observed with increasing depth from ca. 1 m which does not likely reflect the avoidance of deeper areas, but the sampling bias of electrofishing. Although depth was not a significant variable in fyke net samples, we caught nearly similar numbers of pumpkinseed individuals even in depths greater than 2 m, which confirms the sampling bias of electrofishing (Cowx, 1996) (i.e. decreasing visibility of fish) with increasing water depth. We conclude that pumpkinseed avoids very shallow waters (< 0.5 m) in Lake Balaton, and that increasing depth is rather indifferent in the habitat choice of the species. Habitat type has a more influential effect on its distribution than water depth.
Comparisons between active and passive gears are difficult due to several reasons (e.g. different sampling effort, inconstant efficiency with increasing water depth, selectivity for certain species) (e.g. Fago, 1998; Diana et al., 2006; Collins et al., 2015). Although our CPUE data of electrofishing and fyke netting cannot be compared directly with statistical analysis, it was clear that in the configuration we used, electrofishing caught more individuals of both monkey goby and pumpkinseed and habitat use of both species was irrespective of the gear used (i.e. preference to anthropogenically modified habitats). In fact, we believe that beside electrofishing, which is the most commonly used method in the routine monitoring of fish assemblages in the littoral zone of lakes (CEN, 2003), fyke netting could be a complementary method to more precisely describe the habitat-use patterns of our studied and also other fish species, mainly in deeper water, where efficiency of electrofishing is limited (Cowx, 1996; Erős et al., 2008). Moreover, fyke netting can be especially useful where water transparency depends strongly on weather, like in Lake Balaton, as unlike other gears (e.g. visual techniques, such as underwater videos), fyke netting could be effective even in turbid waters (e.g. Knight & Bain, 1996).
Our findings confirm previous studies on other species that fyke nets tend to catch smaller individuals, while electrofishing tends to catch larger individuals in a greater proportion (e.g. Chick et al., 1999; Ruetz et al., 2007; Warry et al., 2013; Francis et al., 2014). Size selectivity of fyke nets may depend on mesh size and throat configuration (Shoup et al., 2003; Ruetz et al., 2007; Smith et al., 2016). Nevertheless, our fyke nets had an expandable 15 cm throat size which was able to allow the entry of larger fish [e.g. the biggest pike-perch Sander lucioperca (Linnaeus, 1758) was 450 mm, and the biggest tench Tinca tinca (Linnaeus, 1758) was 390 mm]. Based on this, larger fish are more likely to avoid fyke nets with small mesh size relative to their body size (Shoup et al., 2003; Ruetz et al., 2007). Moreover, smaller individuals of both monkey goby and pumpkinseed (typically < 60–70 mm in this study) may consider fyke nets as shelters resulting the higher frequency of these size classes collected by this gear type.
Sustainable management of freshwater ecosystems is crucial in the preservation of biodiversity worldwide, and one of the most important aspects in conservation and rehabilitation of these ecosystems is to control the negative impacts of invasive species (Dudgeon et al., 2006; Pelicice et al., 2017). Here, we characterised the habitat preference of two highly invasive fish species, the monkey goby and the pumpkinseed in the littoral zone of Lake Balaton using two different methods, electrofishing and fyke netting. To our knowledge, this study is amongst the first to examine the distribution patterns of monkey goby in lakes. We found that both species preferred anthropogenically modified habitat types (rip-rap shorelines and harbours), the expansion of which may facilitate their spreading. We also found that the two sampling gears provide complementary information on habitat use and length frequency distribution. Thus, multi gear surveys are important in characterising habitat use and population structure more precisely, the knowledge of which is a prerequisite in the management and eradication of invasive species (Verhelst et al., 2015).
References
Adámek, Z., J. Andreji & J. M. Gallardo, 2007. Food habits of four bottom-dwelling gobiid species at the confluence of the Danube and Hron Rivers (South Slovakia). International Review of Hydrobiology 92: 554–563.
Bauer-Haáz, É. A., Á. Ferincz, Z. Szegvári, G. L. Széles & J. Lanszki, 2014. Fish preference of the Eurasian otter (Lutra lutra) on an abandoned fish pond and the role of fish sampling methods. Fundamental and Applied Limnology 184: 161–168.
Bíró, P., 1971. Neogobius fluviatilis in Lake Balaton: a Ponto-Caspian goby new to the fauna of central Europe. Journal of Fish Biology 4: 249–255.
Bíró, P., 1997. Temporal variation in Lake Balaton and its fish populations. Ecology of Freshwater Fish 6: 196–216.
Bíró, P., A. Specziár & K. Keresztessy, 2003. Diversity of fish species assemblages distributed in the drainage area of Lake Balaton (Hungary). Hydrobiologia 506–509: 459–464.
Bonvechio, K. I., R. E. Sawyers, R. Bitz & S. Crawford, 2014. Use of mini-fyke nets for sampling shallow-water fish communities in Florida Lakes. North American Journal of Fisheries Management 34: 693–701.
Borcherding, J., A. Hertel & S. Breiden, 2013. Activity and competitive behaviour of invasive Neogobius melanostomus and Ponticola kessleri (Gobiidae) from the River Rhine, Germany. Ethology Ecology & Evolution 25: 351–365.
Brandner, J., J. Pander, M. Mueller, A. F. Cerwenka & J. Geist, 2013. Effects of sampling techniques on population assessment of invasive round goby Neogobius melanostomus. Journal of Fish Biology 82: 2063–2079.
Byers, J. E., 2002. Impact of non-indigenous species on natives enhanced by anthropogenic alteration of selection regimes. Oikos 97: 449–458.
Čápová, M., I. Zlatnická, V. Kováč & S. Katina, 2008. Ontogenetic variability in the external morphology of monkey goby, Neogobius fluviatilis (Pallas, 1814) and its relevance to invasion potential. Hydrobiologia 607: 17–26.
CEN (European Committee for Standardization), 2003. Water quality: sampling of fish with electricity. Brussels, 18p.
Chick, J. H., S. Coyne & J. C. Trexler, 1999. Effectiveness of airboat electrofishing for sampling fishes in shallow, vegetated habitats. North American Journal of Fisheries Management 19: 957–967.
Ciutti, F., M. E. Beltrami, I. Confortini, S. Cianfanelli & C. Cappelletti, 2011. Non-indigenous invertebrates, fish and macrophytes in Lake Garda (Italy). Journal of Limnology 70: 315–320.
Clark, S. J., R. J. Jackson & S. E. Lochmann, 2007. A comparison of shoreline seines with fyke nets for sampling littoral fish communities in floodplain lakes. North American Journal of Fisheries Management 27: 676–680.
Clavero, M. & E. García-Berthou, 2005. Invasive species are a leading cause of animal extinctions. Trends in Ecology and Evolution 20: 110.
Clavero, M., V. Hermoso, E. Aparicio & F. N. Godinho, 2013. Biodiversity in heavily modified waterbodies: native and introduced fish in Iberian reservoirs. Freshwater Biology 58: 1190–1201.
Cleveland, W. S., E. Grosse & W. M. Shyu, 1992. Local regression models. In Chambers, J. M. & T. J. Hastie (eds), Statistical models in S. Wadsworth & Brooks, Pacific Grove.
Collins, S. F., S. E. Butler, M. J. Diana & D. H. Wahl, 2015. Catch rates and cost effectiveness of entrapment gears for Asian carp: a comparison of pound nets, hoop nets, and fyke nets in backwater lakes of the Illinois River. North American Journal of Fisheries Management 35: 1219–1225.
Cooper, M. J., C. R. Ruetz, D. G. Uzarski & B. M. Shafer, 2009. Habitat use and diet of the round goby (Neogobius melanostomus) in coastal areas of Lake Michigan and Lake Huron. Journal of Freshwater Ecology 24: 477–488.
Copp, G. H., J. R. Britton, Z. Guo, V. R. Edmonds-Brown, J. Pegg, L. Vilizzi & P. I. Davison, 2017. Trophic consequences of non-native pumpkinseed Lepomis gibbosus for native pond fishes. Biological Invasions 19: 25–41.
Cowx, I. G., 1996. Fishing news books. Blackwell Science, Oxford.
Crawley, M. J., 2005. Statistics: an introduction using R. Wiley, Chichester.
Cucherousset, J., G. H. Copp, M. G. Fox, E. Sterud, H. H. Van Kleef, H. Verreycken & E. Záhorská, 2009. Life-history traits and potential invasiveness of introduced pumpkinseed Lepomis gibbosus populations in northwestern Europe. Biological Invasions 11: 2171–2180.
Danylchuk, A. J. & M. G. Fox, 1996. Size- and age-related variation in the seasonal timing of nesting activity, nest characteristics, and female choice of parental male pumpkinseed sunfish (Lepomis gibbosus). Canadian Journal of Zoology 74: 1834–1840.
Dembski, S., G. Masson, P. Wagner & J. C. Pihan, 2008. Habitat use by YOY in the littoral zone of an artificially heated reservoir. International Review of Hydrobiology 93: 243–255.
Diana, C. M., J. L. Jonas & R. M. Claramunt, 2006. A comparison of methods for sampling round goby in rocky littoral areas. North American Journal of Fisheries Management 26: 514–522.
Didenko, A. V., 2013. Gobiids of the Dniprodzerzhynsk reservoir (Dnieper river, Ukraine): distribution and habitat preferences. Acta Ichthyologica et Piscatoria 43: 257–266.
Dudgeon, D., A. H. Arthington, M. O. Gessner, Z. Kawabata, D. J. Knowler, C. Lévȇque, R. J. Naiman, A. Prieur-Richard, D. Soto, M. L. J. Stiassny & C. A. Sullivan, 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews 81: 163–182.
Eggleton, M. A., J. R. Jackson & B. J. Lubinski, 2010. Comparison of gears for sampling littoral-zone fishes in floodplain lakes of the Lower White River, Arkansas. North American Journal of Fisheries Management 30: 928–939.
Erős, T., A. Sevcsik & B. Tóth, 2005. Abundance and night-time habitat use patterns of Ponto-Caspian gobiid species (Pisces, Gobiidae) in the littoral zone of the River Danube, Hungary. Journal of Applied Ichthyology 21: 350–357.
Erős, T., B. Tóth, A. Sevcsik & D. Schmera, 2008. Comparison of fish assemblage diversity in natural and artificial rip-rap habitats in the littoral zone of a large river (River Danube, Hungary). International Review of Hydrobiology 93: 88–105.
Erős, T., A. Specziár & P. Bíró, 2009. Assessing fish assemblages in reed habitats of a large shallow lake: a comparison between gillnetting and electric fishing. Fisheries Research 96: 70–76.
Fago, D., 1998. Comparison of littoral fish assemblages sampled with a mini-fyke net or with a combination of electrofishing and small-mesh seine in Wisconsin Lakes. North American Journal of Fisheries Management 18: 731–738.
Ferincz, Á., Á. Staszny, A. Weiperth, P. Takács, B. Urbányi, L. Vilizzi, G. Paulovits & G. H. Copp, 2016. Risk assessment of non-native fishes in the catchment of the largest Central-European shallow lake (Lake Balaton, Hungary). Hydrobiologia 780: 85–97.
Fox, M. G. & G. H. Copp, 2014. Old world versus new world: life history alterations in a successful invader introduced across Europe. Oecologia 174: 435–446.
Francis, J. T., J. A. Chiotti, J. C. Boase, M. V. Thomas, B. A. Manny & E. F. Roseman, 2014. A description of the nearshore fish communities in the Huron-Erie Corridor using multiple gear types. Journal of Great Lakes Research 40: 52–61.
Gozlan, E. R., 2008. Introduction of non-native freshwater fish: is it all bad? Fish and Fisheries 9: 106–115.
Grabowska, J., D. Pietraszewski & M. Ondračková, 2008. Tubenose goby Proterorhinus marmoratus (Pallas, 1814) has joined three other Ponto-Caspian gobies in the Vistula River (Poland). Aquatic Invasions 3: 261–265.
Hastie, T. & R. Tibshirani, 1986. Generalized additive models: some applications. Statistical Science 1: 297–318.
Hinlo, R., E. Furlan, L. Suitor & D. Gleeson, 2017. Environmental DNA monitoring and management of invasive fish: comparison of eDNA and fyke netting. Management of Biological Invasions 8: 89–100.
Hughes, N. F., 1986. Changes in the feeding biology of the Nile perch, Lates niloticus (L.) (Pisces: Centropomidae), in Lake Victoria, East Africa since its introduction in 1960, and its impact on the native fish community of the Nyanza Gulf. Journal of Fish Biology 29: 541–548.
Ingram, W. M. & E. P. Odum, 1941. Nests and behavior of Lepomis gibbosus (Linnaeus) in Lincoln Pond, Rensselaerville, NewYork. American Midland Naturalist 26: 182–193.
Istvánovics, V., A. Clement, L. Somlyódi, A. Specziár, L. G. Tóth & J. Padisák, 2007. Updating water quality targets for shallow Lake Balaton (Hungary), recovering from eutrophication. Hydrobiologia 581: 305–318.
Janáč, M., K. Roche, L. Šlapanský, M. Polačik & P. Jurajda, 2018. Long-term monitoring of native bullhead and invasive gobiids in the Danubian rip-rap zone. Hydrobiologia 807: 263–275.
Johnson, P. T. J., J. Olden & M. J. Vander Zanden, 2008. Dam invaders: impoundments facilitate biological invasions into freshwaters. Frontiers in Ecology and the Environment 6: 357–363.
Johnson, T. B., M. Allen, L. D. Corkum & V. A. Lee, 2005. Comparison of methods needed to estimate population size of round gobies (Neogobius melanostomus) in western Lake Erie. Journal of Great Lakes Research 31: 78–86.
Knight, J. G. & M. B. Bain, 1996. Sampling fish assemblages in forested floodplain wetlands. Ecology of Freshwater Fish 5: 76–85.
Kornis, M. S., N. Mercado-Silva & M. J. Vander Zanden, 2012. Twenty years of invasion: a review of round goby Neogobius melanostomus biology, spread and ecological implications. Journal of Fish Biology 80: 235–285.
Kovács, M., G. Turcsányi, Z. Tuba, S. E. Wolcsánszky, T. Vásárhelyi, Á. Dely-Draskovics, S. Tóth, A. Koltay, L. Kaszab, P. Szőke & B. Jankó, 1989. The decay of reed in Hungarian lakes. In Salánki, J. & S. Herodek (eds), Conservation and management of lakes. Akadémiai Kiadó, Budapest: 461–470.
Light, T. & M. P. Marchetti, 2007. Distinguishing between invasions and habitat changes as drivers of diversity loss among California’s freshwater fishes. Conservation Biology 21: 434–446.
Minns, C. K., V. W. Cairns, R. G. Randall & J. E. Moore, 1994. An index of biotic integrity for fish assemblages in the littoral zone of Great Lakes’ area of concern. Canadian Journal of Fisheries and Aquatic Sciences 51: 1804–1822.
Mittelbach, G. G., C. W. Osenberg & P. C. Wainwright, 1992. Variation in resource abundance affects diet and feeding morphology in the pumpkinseed sunfish (Lepomis gibbosus). Oecologia 90: 8–13.
Musil, J., P. Jurajda, Z. Adámek, P. Horký & O. Slavík, 2010. Non-native fish introductions in the Czech Republic: species inventory, facts and future perspectives. Journal of Applied Ichthyology 26: 38–45.
Neophitous, C. & A. J. Giapis, 1994. A study of the biology of pumpkinseed (Lepomis gibbosus (L.)) in Lake Kerkini (Greece). Journal of Applied Ichthyology 10: 123–133.
Ogutu-Ohwayo, R., 1990. The decline of the native fishes of lakes Victoria and Kyoga (East Africa) and the impact of introduced species, especially the Nile perch, Lates niloticus, and the Nile tilapia, Oreochromis niloticus. Environmental Biology of Fishes 27: 81–96.
Pelicice, F. M., V. A. Azevedo-Santos, J. R. S. Vitule, M. L. Orsi, D. P. Lima Junior, A. L. B. Magalhaes, P. S. Pompeu, M. Petrere Jr. & A. A. Agostinho, 2017. Neotropical freshwater fishes imperilled by unsustainable policies. Fish and Fisheries 18: 1119–1133.
Pierce, C. L., A. M. Corcoran, A. N. Gronbach, S. Hsia, B. J. Mullarkey & A. J. Schwartzhoff, 2001. Influence of diel period on electrofishing and beach seining assessments of littoral fish assemblages. North American Journal of Fisheries Management 21: 918–926.
R Core Team, 2015. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org.
Rahel, F. J., 2000. Homogenization of fish faunas across the United States. Science 288: 854–856.
Rezsu, E. & A. Specziár, 2006. Ontogenetic diet profiles and size-dependent diet partitioning of ruffe Gymnocephalus cernuus, perch Perca fluviatilis and pumpkinseed Lepomis gibbosus in Lake Balaton. Ecology of Freshwater Fish 15: 339–349.
Ribeiro, V. M., R. R. Braga, V. Abilhoa & J. R. S. Vitule, 2015. Evaluation of three capture techniques for invasive Micropterus salmoides (Lacépéde, 1802) in a Neotropical reservoir: implications for population control and management. Journal of Applied Ichthyology 31: 1127–1129.
Robinson, B. W., D. S. Wilson & A. S. Margosian, 2000. A pluralistic analysis of character release in pumpkinseed sunfish (Lepomis gibbosus). Ecology 81: 2799–2812.
Roche, K. F., M. Janač & P. Jurajda, 2013. A review of Gobiid expansion along the Danube-Rhine corridor: geopolitical change as a driver for invasion. Knowledge and Management of Aquatic Ecosystems. https://doi.org/10.1051/kmae/2013066.
Ruetz, C. R., D. G. Uzarski, D. M. Krueger & E. S. Rutherford, 2007. Sampling a littoral fish assemblage: comparison of small-mesh fyke netting and boat electrofishing. North American Journal of Fisheries Management 27: 825–831.
Shoup, D. E., R. E. Carlson, R. T. Heath & M. W. Kershner, 2003. Comparison of the species composition, catch rate, and length distribution of the catch from trap nets with three different mesh and throat size combinations. North American Journal of Fisheries Management 23: 462–469.
Smith, B. J., B. G. Blackwell, M. R. Wuellner, B. D. S. Graeb & D. W. Willis, 2016. Escapement of fishes from modified fyke nets with differing throat configurations. North American Journal of Fisheries Management 36: 96–103.
Specziár, A., 2010. Fish fauna of Lake Balaton: stock composition, living conditions of fish and directives of the modern utilization of the fish stock. Acta Biologica Debrecina Supplementum oecologica hungarica 23 (Hydrobiol. Monogr. vol. 2): 7–185. (In Hungarian with an English summary).
Specziár, A., Á. I. György & T. Erős, 2013. Within-lake distribution patterns of fish assemblages: the relative roles of spatial, temporal and random environmental factors in assessing fish assemblages using gillnets in a large and shallow temperate lake. Journal of Fish Biology 82: 840–855.
Takács, P., I. Czeglédi, Á. Ferincz, P. Sály, A. Specziár, Z. Vitál, A. Weiperth & T. Erős, 2017. Non-native fish species in Hungarian waters: historical overview, potential sources and recent trends in their distribution. Hydrobiologia 795: 1–22.
Tomeček, J., V. Kováč & S. Katina, 2007. The biological flexibility of the pumpkinseed: a successful colonizer throughout Europe. In Gherardi, F. (ed.), Biological invaders in inland waters: profiles, distribution, and threats. Springer, Dordrecht: 307–336.
Trebitz, A. S., J. R. Kelly, J. C. Hoffman, G. S. Peterson & C. W. West, 2009. Exploiting habitat and gear patterns for efficient detection of rare and non-native benthos and fish in Great Lakes coastal ecosystems. Aquatic Invasions 4: 651–667.
Van Kleef, H., G. van der Velde, R. S. E. W. Leuven & H. Esselink, 2008. Pumpkinseed sunfish (Lepomis gibbosus) invasions facilitated by introductions and nature management strongly reduce macroinvertebrate abundance in isolated water bodies. Biological Invasions 10: 1481–1490.
Verhelst, P., P. Boets, G. Van Thuyne, H. Verreycken, P. L. Goethals & A. M. Mouton, 2015. The distribution of an invasive fish species is highly affected by the presence of native fish species: evidence based on species distribution modelling. Biological Invasions 18: 427–444.
Vila-Gispert, A. & R. Moreno-Amich, 1998. Seasonal abundance and depth distribution of Blennius fluviatilis and introduced Lepomis gibbosus, in Lake Banyoles (Catalonia, Spain). Hydrobiologia 386: 95–101.
Vitule, J. R. S., C. A. Freire & D. Simberloff, 2009. Introduction of non-native freshwater fish can certainly be bad. Fish and Fisheries 10: 98–108.
Warry, F. Y., P. Reich, J. S. Hindell, J. McKenzie & A. Pickworth, 2013. Using new electrofishing technology to amp-up fish sampling in estuarine habitats. Journal of Fish Biology 82: 1119–1137.
Weaver, M. J. & J. J. Magnuson, 1993. Analyses for differentiating littoral fish assemblages with catch data from multiple sampling gears. Transactions of the American Fisheries Society 122: 1111–1119.
Wiesner, C., 2005. New records of non-indigenous gobies (Neogobius spp.) in the Austrian Danube. Journal of Applied Ichthyology 21: 324–327.
Witte, F., T. Goldschmidt, J. Wanink, M. van Ortien, K. Goudswaard, E. Witte-Maas & N. Bouton, 1992. The destruction of an endemic species flock: quantitative data on the decline of the haplochromine cichlids of Lake Victoria. Environmental Biology of Fishes 34: 1–28.
Wood, S. N., 2006. Generalized additive models an introduction with R. Chapman and Hall, Boca Raton.
Zlinszky, A., 2013. Mapping and conservation of the reed wetlands on Lake Balaton. Department of Plant Systematics, Ecology and Theoretical Biology. Budapest, Eötvös Loránd University. PhD.
Acknowledgements
Open access funding provided by MTA Centre for Ecological Research (MTA ÖK). We would like to express our thanks to those who helped in the field work including Árpád Tóth and Géza Dobos. We also thank Andrea Harnos for her advice in statistical analysis. This study was supported by the GINOP-2.3.2.-15-2016-00004 project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Fernando M. Pelicice
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Czeglédi, I., Preiszner, B., Vitál, Z. et al. Habitat use of invasive monkey goby (Neogobius fluviatilis) and pumpkinseed (Lepomis gibbosus) in Lake Balaton (Hungary): a comparison of electrofishing and fyke netting. Hydrobiologia 846, 147–158 (2019). https://doi.org/10.1007/s10750-019-04060-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-04060-9