Abstract
Many freshwater habitats are subject to change through time. Specifically, natural flow regimes are substantially modified by not only seasonal climatic change, but also anthropogenic activity. Consequently, freshwater organisms are exposed to variable flow, potentially altering their behaviour and subsequently parasite transmission amongst social hosts. Here, we investigate the effects of flow conditions on the shoaling behaviour of Trinidadian guppies (Poecilia reticulata), and the resulting transmission of a directly transmitted ectoparasite, Gyrodactylus turnbulli. Shoals exposed to continuous flow exhibited significantly greater G. turnbulli peak intensities and abundance when compared to fish subjected to interrupted, but not no-flow conditions. Parasite transmission rate was greater in shoals exposed to interrupted flow, resulting in parasites becoming more distributed amongst shoal members and thus reducing mean intensity in comparison to continuous flow shoals. Furthermore, as prevalence increased, the distance between shoaling conspecifics increased at greater rates in interrupted and no-flow conditions compared to continuous flow: indicating that in the absence of flowing water, parasitism has a greater effect on shoaling decisions. This data highlights how fish behaviourally respond to variable flow conditions and the implications for parasite transmission.
Similar content being viewed by others
Introduction
Natural variations in precipitation and the seasonal melting of mountainous snowpack can substantially modify freshwater habitats through time. Consequently, freshwater fauna are often exposed to highly variable flow conditions, which will have direct and indirect effects on fish, particularly with respect to behaviour (reviewed in Liao, 2007). Group formation of fish into loosely aggregated shoals or polarized synchronized schools, for example, provides energetic benefits for group members: the magnitude of which can depend on the water velocity to which fish are exposed (Marras et al., 2015; Ashraf et al., 2017). Whilst there are further benefits to group formation, including enhanced predator detection (Magurran, 1990) and increased foraging efficiency (Pitcher & Parrish, 1993), such behaviour can promote parasite transmission between individuals (Barber et al., 2000). For ectoparasites with direct life cycles, the rate of direct transmission is determined by the transmission coefficient ‘β’, which encompasses contact rates between infected and uninfected conspecifics, and the probability that contact results in transmission (Antonlin, 2008; McCallum et al., 2017). Historically, population-based modeling categorized transmission as either density- or frequency dependent, however, it is now recognised that both transmission modes simultaneously promote disease persistence within populations (Ryder et al., 2007; Ferrari et al., 2011; Hu et al., 2013; McCallum et al., 2017). For social species, including shoaling fish, parasite transmission between individuals may not only be affected by population density, but the frequency of contacts between infected and susceptible hosts, allowing parasite persistence even at low-host densities (Johnson & Paull, 2011).
Whilst the effects of temperature on infection risk are well documented (reviewed by Graham & Harrod, 2009; Karvonen et al., 2010), the effects associated with flow modification remain largely overlooked. Of the few studies that have investigated the relationship between flow condition and parasite transmission, the trend has been towards a higher prevalence (the number of hosts infected within a population) and intensity (the number of parasites infecting a host) within populations inhabiting reduced flows (Barker & Cone, 2000; Bodensteiner et al., 2000; Hallett & Bartholomew, 2008). However, it is difficult to disentangle whether this is because of environmental stressors (e.g., high turbidity or hypoxia) increasing the susceptibility of hosts to disease (see Leniham et al., 1999), the poor dispersal capabilities of parasites between hosts in flowing environments (Sousa & Grosholz, 1991; Hallett & Bartholomew, 2008), or if a hosts' energy resource is debilitated by infection (as in Tierney & Farrell, 2004), therefore, prompting them to frequent energetically favourable regions including shallower, slow flowing water.
Alternatively, fish can conserve energy by swimming in a conspecifics wake: a substantial benefit associated with schooling behaviour. Marras et al. (2015) showed how the energy expenditure of grey mullet swimming alone was significantly greater than those in a school, with individuals that trailed conspecifics conserving the most energy. Frequenting reduced flow velocity regions may be particularly beneficial when shoals are exposed to variable flow conditions. However, shoaling tendencies have been observed to diminish in higher flow rates in common minnow (Garner, 1997), three-spined sticklebacks (Sneddon et al., 2006), and zebrafish (Suriyampola et al., 2017). Contrastingly, Hockley et al. (2014) showed that in no-flow conditions, the shoaling tendencies of Trinidadian guppies (Poecilia reticulate Peters, 1859) were significantly reduced compared to those experiencing flowing water, but only following the introduction of Gyrodactylus turnbulli Harris, 1986 infection. This indicated that parasitism may have a greater influence than flow in determining shoaling preferences. However, in this previous study, it was only possible to assess the immediate short-term effect of parasitism on shoaling within a 30-min time frame and not transmission dynamics over several days.
There are over 400 described Gyrodactylus species that are ubiquitous teleost ectoparasites infecting both tropical and temperature hosts (Bakke et al., 2007). In particular, the tropical Trinidadian guppy-Gyrodactylus turnbulli system has been utilised extensively to investigate social behaviour (e.g. Edenbrow et al., 2011), evolutionary ecology (Pérez-Jvostov et al., 2012; Dargent et al., 2013), and epidemiology (Stephenson et al., 2015; Smallbone et al., 2016). The parasite’s direct life cycle, short generation time (ca. 24 h at 25°C; Scott, 1982) and extreme progenesis can result in exponential population growth (Bakke et al., 2007). In practical terms, parasite intensities and transmission can be accurately monitored in situ at repeated time points from the same individual, without harming or killing the host. This host-parasite system is therefore an optimal model for the current study, which aims to assess, over a prolonged period, how G. turnbulli transmission dynamics in shoals of Trinidadian guppies is affected by continuous, interrupted and no-flow conditions.
Materials and methods
Host and parasite origin
The experimental Trinidadian guppies (Poecilia reticulata) used herein were laboratory-reared descendants of a wild population caught from the Lower Aripo River, Trinidad, in 2012. Guppies were initially maintained at Exeter University and transferred to Cardiff University in 2014. Approximately, 30 guppies were housed in each aquaria in mixed sex groups (1:5 male to female ratio), of which male fish were regularly swapped between tanks to minimise the effects of inbreeding. Despite being in captivity for 3 years prior to the experiment, and thus the magnitude of selection pressures otherwise faced in the wild having been relaxed, descendants from this laboratory population typically retain natural behaviours including predatory escape responses (Stephenson & Reynolds, 2016), social decisions (see Hockley et al., 2014; Stephenson & Reynolds, 2016) and infection dynamics (Stephenson et al., 2018). Female guppies (> 3 months) were size matched (± 3.8 mm) into groups of five individuals, representative of a natural shoal size (Croft et al., 2003). Shoals of five female guppies were deemed an appropriate number for an observer to accurately collect data during behavioural observations. Typically, 2–8 individuals have been used in previous behavioural studies (see Jones et al., 2010; Richards et al., 2010; Herbert-Rea et al. 2017). Each group was housed in 6 l aquaria to familiarize and form shoals for a minimum of 14 days (Griffiths & Magurran, 1997). Shoals were maintained under a 12 h light:12 h dark photoperiod at 22–24°C and fed daily on Aquarian® Tropical fish flakes supplemented weekly with live Daphnia magna Straus, 1820 and Artemia. For experimental infections, Gyrodactylus turnbulli (strain Gt3) was used: isolated from and subsequently maintained on ornamental guppies since 1997. This parasite stock is a well established, understood model that has been used to investigate host-parasite interactions for a number of years (e.g., Cable & van Oosterhout, 2007a, b; Faria et al., 2010; Schelkle et al., 2013; Mohammed et al., 2016; Stephenson et al., 2018). In the wild, it takes only one gyrodactylid to successfully infect a host, from which an epidemic within a fish population can arise (Bakke et al., 2007). Many gyrodactylids (and all Gyrodactylus species) demonstrate a viviparous reproductive strategy, and at least the first-born daughter is derived asexually (see Cable & Harris, 2002). Thus, in the wild, it is possible that gyrodactylid populations can be highly inbred, often descending from one individual, as was the case within our culture.
Flume set-up
Transmission experiments were conducted in three identical open-channel re-circulatory flumes, each measuring 150 cm length × 16 cm depth × 20 cm width (Fig. 1). Flumes were filled with dechlorinated water to a depth of 15 cm and maintained at 22–24°C. A 10 cm diameter impeller was attached to a 1hp three-phase 4-pole motor with a maximum shaft speed of 1500 rpm (Machine Mart), wired to a 1.1 kW inverter (RS Components) to control motor speed. The flume bed was set to a longitudinal gradient of 1:1000. At either end of the flume was a 2-cm thick aluminum honeycomb flow straightener with 6.4 mm cell diameter used to remove the secondary flow currents produced from the bends in the recirculatory pipe system. Flow straighteners also prevented fish from entering the impeller region, therefore, restricting shoals to a 100-cm length section (Test Arena; Fig. 1). The flow depth was fixed and maintained at 15 cm throughout all tests. Three flow conditions were tested. A static ‘no-flow’ condition (impeller off) was used as a control. A continuous ‘flow’ condition comprised a depth-averaged velocity of 6 cm s−1 (measured using a Nixon miniature propeller flow meter), which corresponded to a flowrate of 1.9 l s−1, and falls within the range of flow rates recorded within guppy habitats in Trinidad (Kodric-Brown & Nicoletto, 2005). A 12 h flow:12 h no-flow comprised the ‘interrupted flow’ condition (depth-averaged flow velocity 6 cm s−1) and represented the time guppies frequent a shallow refuge to rest and wherein flow is minimal, particularly at night, in habitats with high-predation regimes (Seghers, 1974; Croft et al., 2003). The flow was synchronized with the rooms’ photoperiod so that fish were only exposed to flow during daylight hours.
2D schematic of the open-channel re-circulatory flume used to assess Gyrodactylus turnbulli transmission and shoaling behaviour in Trinidadian guppy shoals for three flow conditions. Top: plan view, and bottom: side view. Fish were retained within the 100-cm-long test arena using 2-cm-thick aluminium honeycomb flow straighteners (6.4 mm cell diameter). Flow depth was maintained at 15 cm and the flume bed slope was 1 in 1000
Preliminary trial of parasite mobility
Preliminary trials assessed whether the recirculartory nature of the flumes facilitated transmission of dislodged specimens of G. turnbulli (hereafter ‘gyrodactylid’). Familiarised shoals of five female guppies were placed into either a continual (n = 3 shoals), interrupted (n = 3 shoals) or no-flow (n = 3 shoals) condition for a 24-h acclimation period, prior to gyrodactylid exposure. Following acclimation, a heavily infected donor guppy was sacrificed and placed in a dark cupboard for ca. 30 min, whereby such conditions encouraged worms to detach from the deceased host and attach to the water film surface (as in Cable et al., 2002). A sterile pipette was then used to transfer 30 individual worms to a watchglass containing 2 mm depth dechlorinated water, which was subsequently placed in the 18 cm terminal end of the flume (Fig. 1). After 1 h, the watchglass was removed and inspected under a dissection microscope to ensure worms had moved into the water column of the flume. The fins and skin of each guppy were screened under 0.02% tricaine methanesulfonate (MS222) sedation 24, 48 and 72 h post-gyrodactylid introduction to confirm if any worms had infected a host. If no gyrodactylids had attached to a host after 72 h (beyond in vitro gyrodactylid survival time; Schelkle et al., 2013), gyrodactylids were presumed dead and the trial was terminated. No ‘dislodged’ gyrodactylid reinfected a host in any treatment, confirming that any parasite transmission observed during subsequent experimental trials would be as a result of host behaviour, and not the experimental setup.
Experimental procedure
Each shoal of five female guppies (n = 17 shoals) was placed into a flume for a 24-h habituation period prior to a 7-day trial. A total of six shoals were trialled in the no-flow condition, five in continuous flow (one shoal excluded due to the escape of two individuals outside of the observational arena) and six in the interrupted flow condition. On Day 0, guppies were removed from the flume and one randomly selected individual was infected with ca. 30 gyrodactylids (range 28–34 worms; no significant difference in starting intensity between treatments: ANOVA: F2,16 = 1.82, P = 0.194). This entailed placing a heavily infected ‘donor’ guppy within close proximity to an anaesthetised recipient guppy [using 0.02% tricaine methansulfonate (MS222)], to allow gyrodactylids to infect the recipient through direct contact. Direct parasite transmission was observed continuously using a dissecting microscope with fibre optic illumination. The remaining four guppies were sham infected by anaesthetising and manipulating under the microscope without exposure to parasites. Each guppy was measured according to its standard length (‘SL’; mm) and profiled by making a detailed diagram of up to five unique spot patterns and/or body markings, enabling identification of individuals at repeated time points to monitor parasite transmission over the 7-day trial. On each screen day, two independent observers confirmed the identity of each individual using the detailed diagrams created at the start of a trial. Guppies were placed in 1 l-dechlorinated water for 1 h while remaining in visual contact to one another, and fed three individuals of Daphnia magna before being returned to the flume. Infection was confirmed the following day (day 1), and each guppy was screened every other day thereafter (days 3, 5 and 7). For each screening, guppies were individually scooped up using a dry plastic container (in doing so preventing indirect parasite transmission via equipment) and isolated in 1 l-dechlorinated water for a maximum of 15 min, whilst remaining in visual contact. Each guppy was anaesthetised and screened to quantify prevalence (the percentage of infected individuals within the shoal), mean intensity (the mean number of parasites found on infected hosts) and abundance (the number of parasites per fish including zero counts). Transmission rate was calculated as the number of new host infections per day.
Observations of shoaling behaviour, including the number of guppies shoaling, maximum shoal size, shoal size and nearest neighbour distance (a standard measure of shoal cohesiveness), were measured at 1-min increments during a 5-min test period, every day at 9:00 am by an observer. This time was selected to coincide with peak guppy activity. Guppies were recorded as shoaling if they were within four body lengths of one another (Pitcher et al., 1983), and the number of guppies in the largest shoal defined the maximum shoal size. A 2-cm2 grid adhered to the side and base of the flume was used to visually estimate the distance between neighbouring guppies whilst shoaling.
Statistical analysis
Analyses were conducted using R statistical software (version 3.1.3, R Development Core Team, 2009). Using the lme4 library (Bates et al., 2013), Generalised linear models (GLMs) were used to investigate the effect of flow condition and fish size on Gyrodactylus turnbulli transmission dynamics. Dependent terms in each model included (1) G. turnbulli mean transmission rate, (2) G. turnbulli maximum prevalence, (3) time to reach maximum prevalence, (4) G. turnbulli maximum intensity, (5) time to reach maximum intensity, and (6) G. turnbulli maximum abundance. Fixed effects in these models included flow condition, shoal mean SL, and G. turnbulli start intensity. Models were fitted with Gaussian error families with identity or inverse link functions. Model structures were selected based on the lowest residual deviance and AIC values.
Generalised linear mixed-models were constructed to assess the influence of parasitism, fish size and flow condition on the shoaling behaviour of Trinidadian guppies. Dependent terms within these ‘global’ models included (6) the number of guppies shoaling, and (7) the mean nearest neighbour distances between shoaling conspecifics. The independent terms included flow condition (no-flow, interrupted or continual flow), G. turnbulli mean intensity and prevalence (as defined by Bush et al., 1997), host mean SL, day and G. turnbulli start intensity. Interaction terms between flow x parasite mean intensity, flow × prevalence and flow × day were also incorporated into the models. To account for repeated measures, shoal number was included as a random term. Models were fitted with Gaussian error structure and log link functions, and model robustness assessed using residual plots.
During model refinement, several equally well-supported models were identified based on comparisons of Akaike’s Information Criterion (AIC). Thus, an information theoretic approach to multi-model inference was employed to assess the relative importance of each independent term in influencing dependent variables within the global GLMMs (following methods in Burnham & Anderson, 2002). As variables were measured on different scales, model parameters within each global model (containing all independent, dependent and random terms) were standardized to a mean of 0 and standard deviation 0.5 using the arm library (Grueber et al., 2011; Gelman & Su, 2013). The ‘dredge’ function within the MuMIn package (Barton, 2013) was then used to generate a set of ‘top’ models, which fell within 2.5 AICc of the best model. Averaged parameter estimates from this top set of models were then calculated using the ‘model.avg’ function, and the relative importance of each parameter generated by summing the Akaike weights across the models in which the parameter occurred (Burnham & Anderson, 2002).
Results
Shoals exposed to interrupted flow exhibited significantly greater gyrodactylid mean transmission rates compared to those in continuous and no-flow conditions (Table 1, Model 1; Fig. 2a). Over time, prevalence increased in all treatments, and a greater mean transmission rate in interrupted conditions resulted in a significantly higher mean peak prevalence of 100% being reached, compared to 92% and 83% in continuous and no-flow conditions, respectively (Table 1, Model 2).
Mean Gyrodactylus turnbulli transmission rate (a), and G. turnbulli mean intensity (b) within shoals exposed to continuous flow (black), interrupted (dark grey), and no-flow (light grey) conditions. Black dots represent outliers; bars the upper and lower limits; the box the first and third quartile with the median. Pairwise comparisons are denoted by a solid black line between treatments, and the level of significance by an asterisk as follows: *P <0.05; **P < 0.005
Significantly higher gyrodactylid mean intensities and abundance were reached in continuous flow compared to interrupted, but not no-flow conditions (Table 1, Models 4 and 6; Fig. 2b). Shoals that had a higher gyrodactylid start intensity also reached higher intensities (Table 1, Model 4). Additionally, there was a positive correlation between the mean SL of a shoal and gyrodactylid mean intensity (Table 1, Model 4). There was no effect of flow condition, a shoals mean standard length or gyrodactylid start intensity on the time taken to reach maximum gyrodactylid prevalence or intensity during the experimental period (Table 1, Models 3 and 5, respectively).
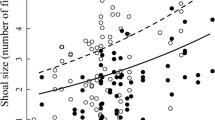
Infection with gyrodactylids had a significant effect on the shoaling behaviour of guppies. As parasite prevalence and intensity increased, the number of guppies shoaling decreased: significantly more so within flowing, compared to interrupted or no-flow conditions (Table 2, Model 6). As parasite prevalence increased, the distances between shoaling conspecifics increased at greater rates in interrupted and no-flow treatments compared to continuous flow conditions (Fig. 3); indicating that in the absence of flowing water, parasitism may have a greater effect on shoaling behaviour (Prevalence × flow interaction: Table 2, Model 7).
The nearest neighbour distances between shoaling conspecifics in relation to Gyrodactylus turnbulli prevalence in continuous (black, n = 5), interrupted (dark grey, n = 6), and no-flow (light grey, n = 6) conditions. Black dots represent outliers; bars the upper and lower limits; the box the first and third quartile with median
Discussion
The current study investigated Gyrodactylus turnbulli transmission dynamics within a social host, the Trinidadian guppy, under prolonged exposure to continuous, interrupted and no-flow conditions. A significantly greater gyrodactylid transmission rate was observed in interrupted flow, resulting in higher parasite prevalence compared to continuous and no-flow conditions. As gyrodactylids reproduce in situ (Cable & Harris, 2002), the transmission potential amongst hosts increases as infrapopulation densities also increase (Bakke et al., 2007). Competition for resources and evasion of a hosts' immune response (see Rubio-Godoy et al., 2012) prompt parasite transmission to new hosts, resulting in the parasite population becoming more evenly distributed throughout a shoal. Within interrupted flow conditions, there may be multiple transmission opportunities. First, through direct contacts as hosts school in flowing water, and, second, during a nocturnal ‘rest’ period when the impeller was switched off. Although guppies are presumed, a diurnal species with shoaling tendencies ceasing as light intensity diminishes (O’Connor & Krause, 2003), nocturnal behaviours, for example prolonged foraging activity, have been observed particularly in the absence of predation (see Fraser et al., 2004). Furthermore, G. turnbulli-infected individuals exhibit nocturnal restlessness (Reynolds & Cable, submitted): frequently encountering and initiating body contact with resting conspecifics during which parasite transmission may occur (Croft et al. 2011). Similarly, for shoals in no-flow conditions, transmission may predominantly occur via nocturnal activity of infected fish moving between resting conspecifics attempting to ‘offload’ their parasite burdens (Reynolds et al., 2017). The success of which, however, may also depend on the behaviour of the ‘recipient’. For example, guppies can discriminate between G. turnbulli-infected and uninfected conspecifics using olfactory cues alone, and respond by initiating evasive behaviours towards infected individuals (Stephenson & Reynolds, 2016). The perception of cues indicative of infection may be enhanced in static compared to flowing environments, prompting fish to avoid infected conspecifics and thus reducing parasite transmission opportunities. This may support why shoals in no-flow conditions had reduced prevalence, compared to those in interrupted and continuous flow conditions.
Maximum gyrodactylid intensity and abundance observed during the study period was significantly greater within continually flowing environments than both interrupted and no-flow conditions and thus a greater transmission rate may have been expected. However, parasites infecting these fish were subject to continuous water flow over the hosts’ body, and although not measured here, coupled with an increased tail beat frequency may have been more vulnerable to dislodgement. Scott & Anderson (1984) estimated that ca. 40% of G. turnbulli became dislodged whilst attempting transmission from donor to recipient fish in static water alone, which is likely to be significantly greater within flowing conditions. Following dislodgement, mortality is inevitable should a parasite fail to encounter a new host, as indicated within our preliminary trials.
A reduced prevalence within continuous flow shoals, compared to interrupted flow shoals, would have resulted in greater mean parasite intensities, whereby a smaller proportion of hosts were infected with the majority of parasites. Additionally, whilst guppies exhibit both innate and acquired immunity towards gyrodactylid infections (Scott, 1985; Cable & van Oosterhout, 2007a), the efficiency of these responses may become compromised if energy reserves are diverted to alternative fitness-related traits, including locomotion (as in Zamora-Camacho et al., 2015; Husak et al., 2016). We speculate that guppies enduring continual flow may divert energy resources into locomotion and consequently compromise immunity to infection, compared to conspecifics experiencing no-flow conditions. This would allow parasite proliferation, particularly on larger hosts which provide more space and resources for parasites. Here, greater parasite intensities were reached in shoals of larger guppies, which is consistent with observations in wild fish, which generally harbour more parasites with maximum parasite loads increasing exponentially with host body size (see Cable & van Oosterhout, 2007b).
As G. turnbulli prevalence and intensity increased, the number of fish shoaling decreased across all treatments, significantly more so within continuous flow conditions. Additionally, we confirm the findings of Richards et al. (2010) that the distances between shoaling guppies increased with parasite prevalence. Shoals became less cohesive at a greater rate within interrupted and no-flow conditions, indicating that in the absence of flowing water, parasitism may have a greater effect on shoaling behaviour. A reduction in shoaling tendency associated with parasitism has been reported in several previous studies (Dugatkin et al., 1994; Krause & Godin, 1996; Hockley et al., 2014), which is not surprising given it is beneficial for individuals to discriminate between infected and uninfected conspecifics, and respond accordingly (see Stephenson & Reynolds, 2016). By remaining within a shoal, but increasing nearest neighbour distances, an individual can simultaneously experience the benefits of swimming in the reduced velocity region of a conspecific’s wake (Marras et al., 2015), whilst also reducing infection risk. When the costs associated with G. turnbulli infection (reviewed in Bakke et al., 2007) outweigh the benefits of remaining with a group, individuals may isolate themselves reducing the overall number of fish shoaling, as observed here. Such isolative sickness behaviour is not uncommon and is demonstrated across multiple taxa (insects; Rueppell et al., 2010, birds; Brown & Brown, 1992, mammals; Proudfoot et al., 2014). Furthermore, exclusion of infected individuals by uninfected conspecifics, and/or the failure of an infected fish in sustaining its shoaling position, particularly within flowing environments (see van Oosterhout et al., 2007), may contribute to a reduction in shoaling tendency.
Conclusions
The specific goal of this study was to identify how variable flow conditions impact parasite transmission dynamics and shoaling behaviour in a social host. Our results highlight how flow alteration substantially modifies parasite transmission dynamics, whereby parasitism was more prevalent in shoals exposed to interrupted flow conditions due to greater transmission rates. Additionally, fish experiencing continuous flow developed intense parasite infections: likely having severe implications for host health. In no-flow conditions, parasitism also appeared to have a greater significance on host shoaling behaviour. It is important to note that only female guppies were used during this experiment due to their greater propensity to shoal than males (Croft et al., 2003). In the wild, male guppies move between female shoals in search of mating opportunities, which could subsequently enhance parasite transmission between individuals (see Richards et al., 2010, 2012). Nevertheless, an understanding of how disease ecology is affected by river flow variability is particularly important considering that parasite infections can substantially modify host population dynamics. Future works should consider extending experimental durations so as to identify peaks in infection parameters and their associated costs for host health and behaviour at a population level.
References
Antonlin, M. F., 2008. Unpacking beta: within-host dynamics and the evolutionary ecology of pathogen transmission. Annual Review of Ecology, Evolution, and Systematics 39: 415–437.
Ashraf, I., H. Bradshaw, T. Ha, J. Halloy, R. Godoy-Diana & B. Thiria, 2017. Simplex phalanx pattern leads to energy saving in cohesive fish schooling. Proceedings of the National Academy of Sciences of the USA 114: 9599–9604.
Bakke, T. A., J. Cable & P. D. Harris, 2007. The biology of gyrodactylid monogeneans: the “Russian- Doll Killers”. Advances in Parasitology 64: 161–376.
Barber, I., D. Hoare & J. Krause, 2000. The effects of parasites on fish behaviour: an evolutionary perspective and review. Reviews in Fish Biology and Fisheries 10: 1–35.
Barker, D. E. & D. K. Cone, 2000. Occurrence of Ergasilus celestis (Copepoda) and Pseudodactylogryrus anguillae (Monogenea) among wild eels (Anguilla rostrata) in relation to stream flow, pH and temperature and recommendations for controlling their transmission among captive eels. Aquaculture 187: 261–274.
Barton, K., 2013. MuMIn: Multi-model inference. R package version 1.9.13. [available on internet at http://CRAN.R-project.org/package = MuMIn].
Bates, D., Maechler, M., Bolker, B., & S. Walker, 2013. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.0-5. [available on internet at http://cran.r-project.org/package=lme4].
Bodensteiner, L. R., R. J. Sheehan, P. S. Wills, A. M. Brandenburg & W. M. Lewis, 2000. Flowing water: an effective treatment for Ichthyophthiriasis. Journal of Aquatic Animal Health 12: 209–219.
Brown, C. R. & M. B. Brown, 1992. Ectoparasitism as a cause of natal dispersal in cliff swallows (Hirundo pyrrhonota). Ecology 73: 1718–1723.
Burnham, K. & D. R. Anderson, 2002. Model selection and multimodel inference. A practical information–theoretic approach, 2nd ed. Springer, New York.
Bush, A. O., K. D. Lafferty, J. M. Lotz, W. Shostak, et al., 1997. Parasitology meets ecology on its own terms: Margolis et al. revisited. Journal of Parasitology 83: 575–583.
Cable, J. & P. D. Harris, 2002. Gyrodactylid developmental biology: historical review, current status and future trends. International Journal for Parasitology 32: 255–280.
Cable, J. & C. van Oosterhout, 2007a. The role of innate and acquired resistance in two natural populations of guppies (Poecilia reticulata) infected with the ectoparasite Gyrodactylus turnbulli. Biological Journal of the Linnean Society 90: 647–655.
Cable, J. & C. van Oosterhout, 2007b. The impact of parasites on the life history evolution of guppies (Poecilia reticulata): the effects of host size on parasite virulence. International Journal for Parasitology 37: 1449–1458.
Cable, J., E. C. Scott, R. C. Tinsley & P. D. Harris, 2002. Behaviour favouring transmission in the viviparous monogenean Gyrodactylus turnbulli. Journal of Parasitology 88: 183–184.
Croft, D. P., B. J. Arrowsmith, J. Bielby, K. Skinnern, E. White, I. D. Couzin, A. E. Magurran, I. Ramnarine & J. Krause, 2003. Mechanisms underlying shoal composition in the Trinidadian guppy, Poecilia reticulata. Oikos 100: 429–438.
Croft, D. P., M. Edenbrow, S. K. Darden, W. Ramnarine, C. van Oosterhout & J. Cable, 2011. Effect of gyrodactylid ectoparasites on host behaviour and social network structure in guppies, Poecilia reticulata. Behavioural Ecology and Sociobiology 65: 2219–2227.
Dargent, F., M. E. Scott, A. P. Hendry & G. F. Fussmann, 2013. Experimental elimination of parasites in nature leads to evolution of increased resistance in hosts. Proceedings of the Royal Society B: Biological Sciences 280: 20132371.
Dugatkin, L. A., G. J. FitzGerald & J. Lavoie, 1994. Juvenile three-spined sticklebacks avoid parasitized conspecifics. Environmental Biology of Fishes 39: 215–218.
Edenbrow, M., S. K. Darden, I. W. Ramnarine, J. P. Evans, R. James & D. P. Croft, 2011. Environmental effects on social interaction networks and male reproductive behaviour in guppies Poecilia reticulata. Animal Behaviour 81: 551–558.
Faria, P. J., C. van Oosterhout & J. Cable, 2010. Optimal release strategies for captive-bred animals in reintroduction programs: experimental infections using the guppy as a model organism. Biological Conservation 143: 35–41.
Ferrari, M. J., S. E. Perkins, L. W. Pomeroy & O. N. Bjørnstad, 2011. Pathogens, social networks, and the paradox of transmission scaling. Interdisciplinary Perspectives on Infectious Diseases 2011: 267049.
Fraser, D. F., J. F. Gilliam, J. T. Akkara, B. W. Albanese & S. B. Snider, 2004. Night feeding by guppies under predator release: effects on growth and daytime courtship. Ecology 85: 312–319.
Garner, P., 1997. Effects of variable discharge on the velocity use and shoaling behaviour of Phoxinus phoxinus. Journal of Fish Biology 50: 1214–1220.
Gelman, A., & Y. S. Su, 2013. arm: Data analysis using regression and multilevel/hierarchical models. R package version 1.6-10. [available on internet at http://cran.r-project.org/package=arm].
Graham, C. T. & C. Harrod, 2009. Implications of climate change for the fishes of the British Isles. Journal of Fish Biology 74: 1143–1205.
Griffiths, S. W. & A. E. Magurran, 1997. Schooling preferences for familiar fish vary with group size in a wild guppy population. Proceedings of the Royal Society B: Biological Sciences 264: 547–551.
Grueber, C. E., S. Nakagawa, R. J. Laws & I. G. Jamieson, 2011. Multimodel inference in ecology and evolution: challenges and solutions. Journal of Evolutionary Biology 24: 699–711.
Hallett, S. L. & J. L. Bartholomew, 2008. Effects of water flow on the infection dynamics of Myxobolus cerebralis. Parasitology 135: 371–384.
Herbert-Rea, J. E., E. Rosén, A. Szorkovsky, C. C. Iannou, B. Rogell, A. Perna, I. W. Ramnarine, A. Kotrschal, N. Kolm, J. Krause & D. J. T. Sumpter, 2017. How predation shapes the social interaction rules of shoaling fish. Proceedings of the Royal Society B, Biological Sciences 284: 20171126.
Hockley, F. A., C. A. M. E. Wilson, N. Graham & J. Cable, 2014. Combined effects of flow condition and parasitism on shoaling behaviour of female guppies Poecilia reticulata. Behavioural Ecology and Sociobiology 68: 1513–1520.
Hu, H., K. Nigmatulina & P. Eckhoff, 2013. The scaling of contact rates with population density for the infectious disease models. Mathematical Biosciences 244: 125–134.
Husak, J. F., H. A. Ferguson & M. B. Lovern, 2016. Trade-offs among locomotor performance, reproduction and immunity in lizards. Functional Ecology 30: 1665–1674.
Johnson, P. T. J. & S. H. Paull, 2011. The ecology and emergence of diseases in fresh waters. Freshwater Biology 56: 38–657.
Jones, K. A., D. P. Croft, I. W. Ramnarine & J.-G. J. Godin, 2010. Size-assortative shoaling in the guppy (Poecilia reticulata): the role of active choice. Ethology 116: 147–154.
Karvonen, A., P. Rintamäki, J. Jokela & E. T. Valtonen, 2010. Increasing water temperature and disease risks in aquatic systems: climate change increases the risk of some, but not all, diseases. International Journal for Parasitology 40: 1483–1488.
Kodric-Brown, A. & P. F. Nicoletto, 2005. Courtship behavior, swimming performance, and microhabitat use of Trinidadian guppies. Environmental Biology of Fishes 73: 299–307.
Krause, J. & J. G. Godin, 1996. Influence of parasitism on shoal choice in the banded killifish (Fundulus diaphanus, Teleostei, Cyprinodontidae). Ethology 102: 40–49.
Leniham, H. S., F. Micheli, S. W. Shelton & C. H. Peterson, 1999. The influence of multiple environmental stressors on susceptibility to parasites: an experimental determination with oysters. Limnology and Oceanography 44: 910–924.
Liao, J. C., 2007. A review of fish swimming mechanics and behaviour in altered flows. Philosophical Transactions of the Royal Society B: Biological Sciences 362: 1973–1993.
Magurran, A. E., 1990. The adaptive significance of schooling as an anti-predator defence in fish. Annales Zoologici Fennici 27: 51–56.
Marras, S., S. S. Killen & J. Lindström, 2015. Fish swimming in schools save energy regardless of their spatial position. Behavioural Ecology and Sociobiology 69: 219–226.
McCallum, H., A. Fendton, P. J. Hudson, B. Lee, B. Levick, R. Normal, S. E. Perkins, M. Viney, A. J. Wilson & J. Lello, 2017. Breaking beta: deconstructing the parasite transmission function. Philosophical Transactions of the Royal Society B: Biological Sciences 372: 20160084.
Mohammed, R. S., M. Reynolds, J. James, C. Williams, A. Mohammed, A. Ramsubhag, C. van Oosterhout & J. Cable, 2016. Getting into hot water: sick guppies frequent warmer thermal conditions. Oecologia 181: 911–917.
O’Connor, E. & J. Krause, 2003. Effect of light intensity on the shoaling behaviour of the guppy (Poecilia reticulata). Journal of Fish Biology 63: 254.
Pérez-Jvostov, F., A. P. Hendry, G. F. Fussmann & M. E. Scott, 2012. Are host-parasite interactions influenced by adaptation to predators? A test with guppies and Gyrodactylus in experimental stream channels. Oecologia 170: 77–88.
Pitcher, T. J. & J. K. Parrish, 1993. Functions of shoaling behaviour in teleosts. In Pitcher, T. J. (ed.), Behaviour of Teleost Fishes. Chapman and Hall, London: 363–439.
Pitcher, T. J., A. E. Magurran & J. R. Allan, 1983. Shifts of behaviour with shoal size in cyprinids. Proceedings of the Brackish and Freshwater Fish Conference 3: 220–228.
Proudfoot, K. L., M. B. Jensen, D. M. Weary & M. A. H. von Keyserlingk, 2014. Dairy cows seek isolation at calving and when ill. Journal of Dairy Science 97: 2731–2739.
R Development Core Team, 2009. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Reynolds, M., & J. Cable, 2017. Ectoparasite infections increase nocturnal activity of their fish hosts. Submitted.
Reynolds, M., A. E. Arapi & J. Cable, 2017. Parasite-mediated host behavioural modifications: Gyrodactylus turnbulli infected Trinidadian guppies increase contact rates with conspecifics. Parasitology 8: 1–7.
Richards, E. L., C. van Oosterhout & J. Cable, 2010. Sex-Specific differences in shoaling affect parasite transmission in guppies. PLoS ONE 5: e13285.
Richards, E. L., C. van Oosterhout & J. Cable, 2012. Interactions between male guppies facilitates the transmission of the monogenean ectoparasite Gyrodactylus turnbulli. Experimental Parasitology 132: 483–486.
Rubio-Godoy, M., G. Muñoz-Córdova, M. Garduño-Lugo, M. Salazar-Ulloa & G. Mercado-Vidal, 2012. Microhabitat use, not temperature, regulates intensity of Gyrodactylus cichlidarum long-term infection on farmed tilapia: are parasites evading competition or immunity? Veterinary Parasitology 183: 305–316.
Rueppell, O., M. K. Hayworth & N. P. Ross, 2010. Altruistic self-removal of health-compromised honeybee workers from their hive. Journal of Evolutionary Biology 23: 1538–1546.
Ryder, J. J., M. R. Miller, A. White, R. J. Knell & M. Boots, 2007. Host-parasite population dynamics under combined frequency- and density-dependent transmission. Oikos 116: 2017–2026.
Schelkle, B., D. Snellgrove & J. Cable, 2013. In vitro and in vivo efficacy of garlic compounds against Gyrodactylus turnbulli infecting the guppy (Poecilia reticulata). Veterinary Parasitology 198: 96–101.
Scott, M. E., 1982. Reproductive potential of Gyrodalyus bullatarudis (Monogenea) on guppies (Poecilia reticulata). Parasitology 85: 217–236.
Scott, M. E., 1985. Dynamics of challenge infections of Gyrodactylus bullatarudis Turnbull (Monogenea) on guppies, Poecilia reticulata (Peters). Journal of Fish Diseases 8: 495–503.
Scott, M. E. & R. M. Anderson, 1984. The population dynamics of Gyrodactylus bullatarudis (Monogenea) within laboratory populations of the fish host Poecilia reticulata. Parasitology 89: 159–194.
Seghers, B. H., 1974. Schooling behavior in the guppy (Poecilia reticulata): an evolutionary response to predation. Evolution 28: 486–489.
Smallbone, W., J. Cable & A. Maceda-Veiga, 2016. Chronic nitrate enrichment decreases severity and induces protection against an infectious disease. Environmental International 91: 265–270.
Sneddon, L. U., S. Hawkesworth, V. A. Braithwaite & J. Yerbury, 2006. Impact of environmental disturbance on the stability and benefits of individual status within dominance hierarchies. Ethology 112: 437–447.
Sousa, W. P. & E. D. Grosholz, 1991. The influence of habitat structure on the transmission of parasites. In Bell, S. S., E. D. McCoy & H. R. Mushinsky (eds.), Habitat Structure: The Physical Arrangement of Objects in Space. Chapman and Hall, London: 300–324.
Stephenson, J. F. & M. Reynolds, 2016. Imprinting can cause a maladaptive preference for infectious conspecifics. Biology Letters 12: 20160020.
Stephenson, J. F., C. van Oosterhout, R. S. Mohammed & J. Cable, 2015. Parasites of Trinidadian guppies: evidence for sex- and age-specific trait-mediated indirect effects of predators. Ecology 96: 489–490.
Stephenson, J. F., S. Perkins & J. Cable, 2018. Transmission risk predicts avoidance of infected conspecifics in Trinidadian guppies. Journal of Animal Ecology 87: 1525–1533.
Suriyampola, P. S., D. J. Sykes, A. Khemka, D. S. Shelton & E. P. Martins, 2017. Water flow impacts group behaviour in zebrafish (Danio rerio). Behavioural Ecology 28: 94–100.
Tierney, K. B. & A. P. Farrell, 2004. The relationships between fish health, metabolic rate, swimming performance and recovery in return-run sockeye salmon, Oncorhynchus nerka (Walbaum). Journal of Fish Diseases 27: 663–671.
van Oosterhout, C., R. S. Mohammed, H. Hansen, G. A. Archard, M. McMullan, D. J. Weese & J. Cable, 2007. Selection by parasites in spate conditions in wild Trinidadian guppies (Poecilia reticulata). International Journal for Parasitology 37: 805–812.
Zamora-Camacho, F. J., S. Reguera, M. V. Rubiño-Hispán & G. Moreno-Rueda, 2015. Eliciting an immune response reduces sprint speed in a lizard. Behavioural Ecology 26: 115–120.
Acknowledgements
We thank Darren Croft for providing the original fish and Zach Smallbone and Grace Dugdale for technical assistance.
Funding
FH was funded by a BBSRC studentship with CASE funding from the Centre for Environment, Fisheries and Aquaculture Science (Cefas) BB/F016557/1.
Author information
Authors and Affiliations
Contributions
FH and JC designed the study; CAMEW provided technical advice; MR and FH collected the data; MR performed statistical analysis and drafted the manuscript; all authors commented on manuscript drafts.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional and/or national guidelines for the care and use of animals were followed. Procedures and protocols were conducted under UK Home Office license (PPL 303424) with approval by the Cardiff University Animal Ethics Committee.
Additional information
Handling editor: Checo Colón-Gaud
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Reynolds, M., Hockley, F.A., Wilson, C.A.M.E. et al. Assessing the effects of water flow rate on parasite transmission amongst a social host. Hydrobiologia 830, 201–212 (2019). https://doi.org/10.1007/s10750-018-3863-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-018-3863-x