Abstract
Since phytoplankton production is usually estimated from static incubations (fixed depths or light levels), a mesocosm study was performed to evaluate the significance of mixing depth, mixing intensity and load of humus of natural phytoplankton assemblages. Vertically rotated (dynamic) incubations usually gave higher results than static incubations in humus-rich water. Mixing intensity was of significant importance in one of 2 years tested, but strong interaction effects with humus complicated the explanation. Differences in primary production between dynamic incubations did not fully reflect the received PAR dose, and increased humus and increased mixing depth increased the photo-assimilation efficiency. Different single-depth incubations did not provide a shortcut method to measure water-column primary production with high accuracy. Results diverged from theoretical estimates based on recent combined photo-biological and physical environmental models. The large variability in responses to mixing is supposed to reflect species-specific adaptations and pre-history regarding quantity (photons) and quality (spectral distribution) of the optical environment in an assemblage of different species. The proportional abundance of each species with its specific characters will therefore strongly influence bulk primary production. Due to such variable responses, clear guidelines for a “best practice” in primary production measurements cannot be given, based on the present results.
Similar content being viewed by others
Introduction
Measuring primary production in the field includes trapping a bulk sample of the phytoplankton community in transparent bottles and incubating for a given time, using a series of fixed depths or irradiance levels or by using on-deck incubators that provide a range of different irradiance levels, comparable to a vertical profile of the in situ irradiance levels. In the natural environment, phytoplankton in the upper mixed layer are probably experiencing variable light conditions that are poorly mirrored by phytoplankton held in bottles at fixed depth or in incubators. Since phytoplankton photosynthesis is mediated by physiological adaptations to different irradiance levels (Falkowski, 1983), their responses to vertical mixing will depend primarily on the intensity of mixing. Since the spectral distribution changes with depth and depends on the content of e.g. humic substances, a deck incubator with neutral filters for different irradiance levels will not properly reproduce the light in a natural water column (Walsh & Legendre, 1983; Bertoni & Balseiro, 2005). In nature, phytoplankton cells may constantly change their depth in a random manner by turbulent mixing e.g. due to tidal mixing (Delgadillo-Hinojosa et al., 1997; Lizon et al., 1998) or in a regular manner by internal waves and Langmuir circulation (Denman & Gargett, 1983; Farmer & McNeil, 1999). Phytoplankton cells are therefore usually experiencing a variable optical environment, regarding both intensity and spectral composition. Due to continuous turbulent mixing, a water sample taken from a single depth will contain algal cells with different light histories and thus different photo-acclimation states (Ross et al., 2011a).
Attempts have been made to evaluate if a variable light environment is of significance for algal photosynthesis by using laboratory studies on single species as well as field studies on natural assemblages (e.g. Marra, 1978a, b; Gallegos & Platt, 1982; Yoder & Bishop, 1985; Kromkamp & Limbeek, 1993; Helbling et al., 2003, 2013; Bertoni & Balseiro, 2005; Bertoni et al., 2011; Gali et al., 2013; Lawrenz & Richardson, 2017). Results indicate variable effects of fluctuating irradiance, among other things related to turbidity (e.g. Helbling et al., 2013), temperature (Edwards et al. 2016), day-to-day variability (Bertoni et al., 2011) and species differences (e.g. Lawrenz & Richardson, 2017). More theoretical evaluations have also been made to evaluate the significance of water-column mixing on growth and production of microalgae, using mathematical simulation models where photo-biological functions are combined with physical environmental functions (Falkowski & Wirick, 1981; Gallegos & Platt, 1985; Patterson, 1991; Tirok & Gaedke, 2007; Ross et al., 2011a, b). The models are either based on the average values of photosynthetic properties of the natural assemblage of cells in relation to mixing properties (bulk property model, Lizon et al., 1998) or based on physiological responses of individual phytoplankton cells to vertical displacements (Lagrangian model, Lizon et al., 1998; Ross et al., 2011a). The model by Ross et al., (2011a) represents the most comprehensive one, where random particle movements in a vertical plane are combined with a physiological model with over 20 input variables.
Attempts have also been made to construct mechanical devices for dynamic measurements of primary production. Gocke & Lenz (2004) constructed a deck incubator where the incubated water samples rotated underneath 11 neutral filter sections, approximately simulating the illumination of an algal cell moving from the surface to the depth of 1% surface irradiance. Higher primary production was measured with this device compared to static in situ incubations at 9 out of 11 test sites, with the most pronounced differences recorded for the most turbid environments. However, neutral filters do not reproduce the natural change in spectral distribution with depth, and a more advanced in situ device, as the ones presented by Köhler (1997) and Bertoni & Balseiro (2005) would therefore be a better choice. However, none of these two studies provide sufficient results for an evaluation of dynamic versus static incubations, since none of them present results for integrated water-column production of static incubations.
The response of an algal cell to different irradiance levels is described by the photosynthesis/irradiance (P/I) relationship, which has a form of a hyperbolic function with an initial slope and an asymptotic upper level that is specific for each species or genotype with its own pre-history and environmental adaptations. In addition there migt also be a negative effect on photosynthesis of high irradiance and ultraviolet radiation, also that highly specific for different species or genotypes. With an assemblage of different species and genotypes that makes up a natural phytoplankton community, the community P/I relationship and physiological responses to a variable optical environment may therefore not reflect a simple P/I relationship and responses as shown by a single species or genotype.
A key question, in understanding how vertical mixing and thereby variable irradiance affects the photosynthesis, is how fast the algal cells respond to changes in irradiance. A review by Ferris & Christian (1991) shows that adaptations to high levels are usually immediate or occur within minutes, whereas adaptations to low light appear to be slower. However, the literature does not provide strong evidences for when and how variable irradiance governs primary production. Lizon et al. (1998), used a Lagrangian random-walk model to study the interactions between periodic vertical tidal mixing and primary production of phytoplankton in the eastern English Channel, and found that reduced mixing between spring and neap tide caused increased primary production. Reduced primary production caused by mixing was earlier reported for turbid waters where the ratio between euphotic and mixed depth was low (Randall & Day, 1987; Grobbelaar, 1989). Yoder & Bishop (1985) on the other hand, found no differences in primary production between vertically cycled incubations and the production as defined by the average irradiance and the P/I curve as given by static incubations. Joiris & Bertels (1985) found that in vitro incubations under fluctuating light conditions and in situ incubations under varying depth provided higher primary production at low light intensities, both with monocultures and with natural populations. Havelkova-Dousova et al. (2004) used the green alga Dunaliella tertiolecta Butcher in static and dynamic irradiance regimes and the growth in the dynamic regime was much higher than growth in the static regime at the same average irradiance. Laboratory studies on the marine diatom Skeletonema costatum (Greville) Cleve showed a clear adaptation to a fluctuating light climate by decreasing the size and increasing the number of its photosynthetic units, thereby also increasing its maximum photo-assimilation (Kromkamp & Limbeek, 1993). Lawrenz & Richardson (2017) working in a blackwater environment (rapid light absorption with depth), showed that S. costatum and the cryptophyte Rhodomonas salina (Wislouch) Hill & Wetherbee had very different photo-acclimation strategy but that both species enhanced growth rate and primary productivity when experiencing periods of full spectrum light.
In the present paper, I have used an indoor mesocosm facility for experiments in water with high turbidity, corresponding to coastal areas with outflow of river water with high content of humus material or lakes and ponds with brownified water from the surroundings. In such environments, the light level will decrease rapidly with depth, and therefore, vertical mixing will make phytoplankton cells experiencing large variations in irradiance. I also evaluate if measurements at a single depth, based either on the extent of the mixed layer or on the vertical irradiance profile, could replace a series of measurements at different depths.
Materials and methods
The mesocosm facility
Two experimental series were performed, one in April 2013 and one in April 2014 in the indoor mesocosm facility at Umeå Marine Sciences Centre, University of Umeå, Sweden, situated at the northern Bothnian Sea (N63°34′; E19°50′) in the Baltic Sea. The facility is described by Båmstedt & Larsson (2018). For the experiments, I used two mesocosm tanks, 5 m high and 0.73 m in diameter, filled with pre-filtered (300 μm porosity) brackish water of salinity 4.3, taken from 2 m depth. In 2013, the two tanks were filled on 27th of March and experiments started on the 4th of April. In 2014, the tanks were filled on the 2nd of April and experiments started on the 7th of April. One tank was used for maintaining the natural plankton community and taking out water samples for the experiments, the other one was used for incubations of the water samples. The temperature was held at 15 ± 0.2°C, and the whole water column was mixed by warming the lowest temperature mantle, 3.6–5 m in depth. In order to prevent surface heating from the lamp, the upper 0.6 m was slowly bubbled with air (see Båmstedt & Larsson, 2018). Nutrients (nitrate, ammonium, phosphate) were added, sufficient for saturated conditions throughout each experiment, and measurements were started 3–4 days after nutrient additions. Illumination (17 h on, 7 h off) was provided by a metal halogen lamp (Philips MH/CDM-T150 W/930) with an emission spectrum resembling that of natural sunlight, giving around 400 µmol photons m−2 s−1 immediately below the surface.
Phytoplankton community and field temperature at the time of experiments
In the end of March 2013, the phytoplankton was dominated by dinoflagellates (Dinophycea) such as Peridiniella catenata (Levander) Balech (58.38 mg/m3 wet weight), Scrippsiella spp. Balech ex Loeblich III (52.55 mg/m3), Peridinium spp Ehrenberg (39.17 mg/m3), whereas the dominating diatom (Bacillariophycea) was Thalassiosira baltica (Grunow) Ostenfeld with 14.59 mg/m3. The phytoplankton composition was different in the beginning of April 2014, with the diatoms Thalassiosira baltica (87.56 mg/m3 wet weight), Melosira arctica Dickie (29.28 mg/m3), and unidentified centric diatoms (49.20 mg/m3), and with Gymnodinium corrolarium Sundström, Kremp and Daugbjerg (13.67 mg/m3), Scrippsiella spp (7.37 mg/m3) and Protoperidinium spp Bergh ex Loebl. and A.R. Loebl. (5.64 mg/m3) as the dominating dinoflagellates. The field temperature in the upper 5 m water was 0°C in the end of March 2013 and 1.2°C in the beginning of April 2014.
Primary production method
Primary production was measured by incubating 14C-labelled experimental water containing 40 mCi of Na14CO3 L−1 in 20-ml glass incubation vials for 120 min. Two series were used with rotating incubations, hereafter called dynamic incubations. These comprised five replicate incubation vials each, and one series was fixed to a 3-m rubber loop, the other one to a 9-m rubber loop, rotating from the surface to 1.5 m, respectively, 4.5 m in depth and further referred to as shallow (S) and deep (D). The rotation speed could be set arbitrarily, and I used three different speed intervals, 0.6–2.4 cm s−1 (125–180 s revolution−1 and 375–540 s revolution−1 for, respectively, shallow and deep mixed layer), 5.2–6.3 cm s−1 (48–58 s, respectively, 143–173 s revolution−1) and 12.2–13.2 cm s−1 (23–25 s, respectively, 68–74 s revolution−1), further referred to as, respectively, slow (s), medium (m) and high (h) speed. Three different levels of humus material were used. In 2013, the addition was a laboratory grade humic acid (Aldrich pnr: 536080), given in 4 mg l−1 (medium humus) final concentration and 8 mg l−1 (high humus), together with no addition treatment (low humus). In 2014, pure humic acid was replaced by earth extract dissolved in distilled water, given in additions of 800 ml (medium humus) and 2,400 ml (high humus). Although not separately analysed as final humus concentration, measurements of turbidity and the light attenuation coefficient (see later) provided a good indication of its effect on the optical environment. A schematic picture of the rotation incubations is given in Fig. 1. Without humus addition, incubation bottles passing close to the surface will have an irradiance level close to Pmax (Photosynthetic maximum), whereas medium and high humus will reduce the light also close to the surface. The deep rotation also differs from the shallow one by spending only 1/3 of the time compared to the shallow rotation above 1.5 m depth. Average experienced light level will therefore differ considerably between the two rotation series. In order to evaluate effects of pre-condition of the phytoplankton community when experiencing a darker environment, the experimental protocol differed somewhat between the 2 years. In April 2013, I made the same additions of humic acid in the tank for water samples and the incubation tank, whereas in April 2014, humus was only added to the incubation tank. Simultaneously with the dynamic incubations, I used incubations at fixed depths, hereafter called static incubations. In April 2013, I used incubations at eight depths, 0.1, 0.2, 0.4, 0.6, 1.0, 2.0, 3.0 and 4.0 m in depth, and in April 2014, incubations at seven depths, 0.4, 0.8, 1.2, 1.6, 2.0, 2.4 and 2.8 m in depth. I also used two incubation vials for each series as blanks, kept in darkness at the same temperature as the main series and treated as the other samples. In the static incubations without addition of humus, I used two replicates per depth but only the averages of these for each depth were used in the calculations. In all other experiments, I used single incubations at each depth.
Primary production analysis
After incubation, 5.0 ml of the incubated water was transferred to a pre-labelled plastic scintillation vial, 300 μl of 3 M HCl was added, and the samples bubbled with air for 30 min. A scintillation cocktail (Optiphase HiSafe 3, 15 ml) was thereafter added, and the content was mixed and measured in a Beckman 6500 scintillation counter. The value, subtracted by the value for the dark incubations, gave the value for DPMsample (DPM = disintegrations per minute). For each experimental series, I prepared a standard series from the labelled water by pipetting out triplicates of 100, 200, 300 and 400 µl of the labelled water into 5 ml of distilled water. Immediately thereafter, 15 ml of scintillation cocktail was added, the content was mixed and measured as above. This gave the radioactivity added in a 5 ml water sample, expressed as DPMtotal. The coefficient of variation of the average value was always less than 2% for all standard series. Carbon assimilation was then calculated from the formula below:
where t is the incubation time in hours, 1.10 is sum CO2 in the water, given by the total alkalinity times the F-factor, as tabulated in Gargas (1975), 1.05 is a constant compensating for slower uptake of 14C compared to 12C, 1.06 is a constant correction for respiration loss during incubation, 200 is a constant for conversion from production in 5 ml to 1 l and 12 is a constant for converting from mmol C to mg C. See Colijn & Edler (1998) for further description. Primary production per m2 and hour for rotating incubations was obtained by multiplying primary production per m3 and hour by the rotating depth (i.e. 1.5 or 4.5 m). The following procedure was used when calculating primary production from the static incubations. First, the cumulative production m−2 over the incubation depths was recorded (Eq. 7 in Table 1). Thereafter, these results were plotted against depth, and a logarithmic function adapted to the data, describing water-column production m−2 versus depth (Eq. 9 in Table 1). The coefficient of determination (R2) ranged between 0.894 and 0.999 in April 2013 and between 0.878 and 0.999 in April 2014. Then the data given by Eq. (7) in Table 1 were divided with the corresponding depths, then giving the average production m−3 for different water-column depths (Eq. 8 in Table 1). These results were finally used for a regression plot of average production m−3 versus water-column depth and an exponential regression equation calculated (Eq. 10 in Table 1), which gave coefficients of variation ranging from 0.889 to 0.990 in April 2013 and from 0.833 to 0.998 in April 2014. The production by dynamic incubations was considered significantly different from production by fixed incubations when the 95% confidence interval of dynamic incubations did not overlap the calculated production from static incubations. ANOVA tests were ran to evaluate any significant effects of humus additions and different mixing speeds on primary production, then using the statistical package Systat 13 (version 13.1; www.systat.com).
Additional water-column measurements
For profiling of light (PAR, photosynthetic active radiation, 400–700 nm), chlorophyll a, and turbidity (FTU, Formazine Turbidity Units), I used a logging instrument, Aanderaa SeaGuard (see www.aanderaa.com) with simultaneous logging also of pressure (depth). The PAR attenuation coefficient (k) was given by the negative exponent in the exponential equation describing absolute irradiance versus depth. An average PAR dose was calculated for the dynamic incubations as exp[(ln(I1) + ln(I2))/2] × t, where I1 and I2 are the irradiance close to the surface and at the mixing depth (i.e. at 1.5, respectively, 4.5 m), and t is the incubation time (7,200 s). The chlorophyll content and the calculated PAR dose were also used to calculate a standardised primary production for the dynamic incubations, then given as mg C mg Chl. a−1 mol photons−1. The light spectrum was measured in the “low humus” and “medium humus” treatment in 2014, using Ocean Optics™ radiometers USB 2000+ (Ocean Optics, Winter Park, Florida, USA), with the fibre-optical cable mounted on a frame that held the tip of the fibre upward in a vertical position. The instrument was calibrated using the Ocean Optics™ calibration unit, HAL 2000, before each series of measurements. The change in spectrum after addition of humus was displayed as the percentage remaining over the PAR spectrum. In addition to the direct calculations of primary production for static incubations, I also calculated production (i) at mean arithmetic depth, AD; (ii) at mean logarithmic depth, LD; (iii) at depth of mean arithmetic light, DAL; and (iv) at depth of mean logarithmic light, DLL. The actual equation for production per m3 versus depth for each condition was used and multiplied with 1.5 or 4.5 (water column mixing depths). The equations used in the calculations are summarised in Table 1.
The average primary production per m3 for dynamic incubations (two mixing depths and three mixing rates) was used as input values in the equation describing depth as a function of primary production, i.e. depth as the dependent and primary production as the independent variable, derived from the static incubations (Eq. 11 in Table 1). This gave the corresponding depths for each of the average production of the six different combinations of mixing depths and mixing rates. These results are presented together with the calculated depth at average production for 0–1.5 m and 0–4.5 m water column, as given by the static incubations.
Results
The euphotic zone, here defined as the depth of 1% surface irradiance (cf Kirk, 1994), differed between the 2 years in the experiments with no humus addition, where the whole water column was above the euphotic zone in 2013, but 1/5 of the water column received less than 1% of the surface irradiance in 2014 (Table 2). With addition of humus, the euphotic zone was reduced to approximately the same depth in both years, with around 40% of the water column below the euphotic zone with medium humus and around 60% below the euphotic zone with high humus (Table 2). The PAR dose received by the dynamic incubations was usually slightly higher in 2014 than in 2013 (Table 2). Humus additions increased the difference dramatically between shallow and deep mixing. In 2013, the PAR dose in the shallow mixed layer was 3.9 times higher than in the deep mixed layer, and this factor increased to 14.5 times for medium humus and 33.3 times for high humus. Corresponding factors in 2014 were 5.3 times for no humus, 11.4 times for medium humus and 21.0 times for high humus.
Figure 2 shows that the spectral distribution changed markedly with addition of humus in the water. The effect increased with depth and was strongest at short PAR wavelengths. Close to the surface, 40% remained at around 400 nm and this increased slowly to 64% near 700 nm wavelength (Fig. 2). At 3 and 4 m depth, virtually no light remained below 500 nm, but a rapid increase occurred above 600 nm (Fig. 2).
Experiments April 2013
The chlorophyll concentration ranged from 6.1 to 7.8 µg l−1 and the PAR attenuation coefficient in the three different humus additions was 0.90, 1.76 and 2.40, respectively (Fig. 3). Whereas humus additions gave gradually lower production values from the static incubations, dynamic incubations rather showed a tendency of increased production with humus additions (Fig. 3). With no humus addition production per m3 and per m2, both in the upper 1.5-m and the 4.5-m water column were significantly lower than for static incubations. Shallow mixing showed a gradual production increase with increased mixing speed, whereas deep mixing gave highest production with medium mixing speed (Fig. 3A, B). With medium humus and a light attenuation coefficient of 1.76, both shallow and deep mixing showed higher production than static incubations, except treatment Sh and production per m2, and there was also a strong tendency of decreased production with increased mixing speed (Fig. 3C, D). With high humus, and a light attenuation coefficient of 2.40, the dynamic incubations gave production values usually several hundred percent higher than for static incubations (Fig. 3E, F).
Experiment April 2013. Ss shallow orbit (0–1.5 m) and slow speed (0.02 m s−1); Ds deep orbit (0–4.5 m), slow speed; Sm shallow orbit and medium speed (0.05 m s−1); Dm deep orbit, medium speed; Sh shallow orbit, high speed (0.12 m s−1); Dh deep orbit, high speed. A, B No humus added; C, D 4 mg humus l−1 added; E, F 8 mg humus l−1 added. A, C and E display the production per m3, B, D and F display the production per m2. The two horizontal lines in the graphs show the results for incubations at fixed depths, integrated over, respectively, 0–1.5 m (solid lines) and 0–4.5 m depth (broken lines). The PAR irradiance attenuation coefficient (k) and average chlorophyll a concentration (in µg l−1) are displayed in the upper panels. Vertical lines attached to the bars denote 95% confidence intervals (n = 5)
Experiments April 2014
The chlorophyll concentration ranged from 2.7 to 4.1 µg l−1 and light attenuation coefficient from 1.28 to 2.52 m−1. The humus additions decreased the production for static incubations, whereas dynamic incubations showed more variable results (Fig. 4). With no addition of humus, treatments Sm, Sh, Dm and Dh gave significantly lower primary production per m3 and m2 compared to the shallow and deep static incubations (Fig. 4A, B). Medium humus gave significantly lower results for treatments Sm and Dh compared to static incubations, both regarding production per m3 and per m2 (Fig. 4C, D). With additional humus and a PAR attenuation coefficient of 2.52 and turbidity of 2.20 FTU, dynamic incubations gave both higher (Ds) and lower (Sh, Dh) results than static incubations (Fig. 4E, F).
Experiment April 2014. Ss shallow orbit (0–1.5 m) and slow speed (0.02 m s−1); Ds deep orbit (0–4.5 m), slow speed; Sm = shallow orbit and medium speed (0.05 m s−1); Dm deep orbit, medium speed; Sh = shallow orbit, high speed (0.12 m s−1); Dh deep orbit, high speed. A, B No humus added; C, D 800 ml earth extract in distilled water added; E, F 2400 ml earth extract added. A, C and E display the production per m3, B, D and F display the production per m2. The two horizontal lines in the graphs show the results for incubations at fixed depths, integrated over, respectively, 0–1.5 m (solid lines) and 0–4.5 m depth (broken lines). The PAR irradiance attenuation coefficient (k), average chlorophyll a concentration (in µg l−1) and turbidity (mean FTU ± 95% confidence limits) are displayed in the upper panels. Vertical lines attached to the bars denote 95% confidence intervals (n = 5)
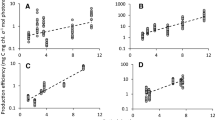
Water-column production comparisons
The calculations of standardised production (see Table 3) showed a slight increase with increased humus addition for the shallow incubations. In April 2013, from between 0.40 and 0.69 mg C mg chl.−1 mol photons−1 with no humus to 1.67–3.49 mg C mg chl.−1 mol photons−1 with high humus, and in April 2014, from 1.63–2.68 to 6.45–11.8 mg C mg chl.−1 mol photons−1 (Table 3). For the deep incubations, the effect of humus addition was more dramatic, from between 1.19 and 1.71 mg C mg chl.−1 mol photons−1 with no humus to between 44.8 and 140.4 mg C mg chl.−1 mol photons−1 with high humus in April 2013 and from 2.06 to 9.90 mg C mg chl.−1 mol photons−1 with no humus to 50.7–178.4 mg C mg chl.−1 mol photons−1 with high humus in April 2014 (Table 3).
Figure 5 summarises the comparison of production estimates based on the various depth parameters defined in the method section and Eqs. (1, 2, 5 and 6) in Table 1. In 2013, the integration method showed a gradual reduction with added humus, from 3.9 mg C m−2 h−1 with no addition, to 0.7 mg C m−2 h−1 for high humus addition for the deep mixed layer (Fig. 5A), and from 2.1 to 0.3 mg C m−2 h−1 for shallow mixed layer (Fig. 5B). With no humus addition, all methods based on the static incubations gave approximately the same production as for the integration method for the deep mixed layer, and these were all higher than the result for dynamic incubations. With medium humus, average arithmetic depth and logarithmic depth (AD and LD) and depth at average logarithmic light (AD, LD and DLL) gave slightly lower results than the integration method, whereas depth of average arithmetic light (DAL) gave slightly higher result, all being considerably lower than the results from dynamic incubations (Fig. 5A). High humus gave roughly the same results for integration and average arithmetic and logarithmic depth (AD and LD) and depth of average arithmetic and logarithmic light (DAL and DLL), and these were all much lower than the results for dynamic incubations (Fig. 5A).
A and B. Water-column primary production, April 2013, calculated by depth integration, using the actual equation for production (mg C m−3 h−1) versus depth from the static incubations (labelled Integration) and by using four calculated depths (See Table 1), as labelled in the figures, in the same equation, multiplied by 4.5 (A deep mixed surface layer), respectively, 1.5 (B shallow mixed surface layer). C and D show the same data for April 2014. The six treatments Ss through Dh represent the dynamic incubations with shallow (S) or deep (D) mixing and slow (s), medium (m) or high (h) mixing speed. Vertical bars denote 95% confidence intervals (n = 5)
Results for the shallow mixed layer with no additional humus showed higher integrated production than the other static incubations, which in turn were quite similar to the dynamic incubations (Fig. 5B). With medium and high humus added, the same tendency was shown as for the deep mixed layer, with relatively small differences between integration and single-depth incubations and a much higher production from the dynamic incubations (Fig. 5B) In 2014, the depth-integrated production decreased gradually with added humus, although less steep than the year before (Fig. 5C, D), in accordance with a smaller span in the PAR attenuation coefficient (k). Results for the deep mixed layer showed rather small effects of humus for average arithmetic and logarithmic depth (AD and LD) and depth of average arithmetic light (DAL), whereas depth of average logarithmic light (DLL) decreased from no humus addition to medium and high humus addition (Fig. 5C). Depth of average arithmetic light (DAL) was higher than integration, whereas average logarithmic depth (LD) and especially average arithmetic depth (AD) gave lower results. The dynamic incubations showed both higher and lower results compared to integration, without any systematic trends (Fig. 5C). For the shallow mixed layer with no humus addition, all estimates from static incubations gave similar results as integration, but with both levels of added humus, the four single-depth estimates were somewhat lower than the integration (Fig. 5D). The dynamic incubations showed both higher and lower results compared to integration (Fig. 5D).
Figure 6A and B shows the depth at which the production m−3 corresponded to the average production for the actual water column (4.5 or 1.5 m) as given by the static and dynamic incubations. In experiments 2013, (Fig. 6A) integration showed only small differences in response to humus addition, with a range from 1.2 m (high humus) to 1.5 m (no humus) for 0–4.5 m depth, and from 0.7 m (no and high humus) to 1.0 m (medium humus) for 0-1.5 m depth. In experiments 2014, the range for 0–4.5 m depth was from 1.6 m (medium and high humus) to 2.0 m (no humus), and from 0.9 m (medium humus) to 1.4 m (no humus) for 0–1.5 m depth (Fig. 6B). Dynamic incubations gave much higher variability, related to different mixing rates and different humus content in the water. In 2013, each of the three mixing rates for deep and shallow mixing showed reduced production depth with increasing humus, where for example deep and slow mixing (Ds) showed reduction from 2.9 m (no humus) to 1.2 m (medium humus) and to 0 m with high humus (Fig. 6A). Similar gradual reductions in corresponding production depths were shown for shallow and slow mixing (Ss), with the values 2.4, 1.0 and 0 m (Fig. 6A). The results were different in 2014, with only medium mixing rate for deep and shallow mixing (Dm and Sm) showing a gradual decrease in corresponding production depth with increased humus content (Fig. 6B). The other mixing schemes showed either roughly constant production depth (Dh: 1.6–2.0 m, Ss: 0.7–0.9 m), highest (Ds) at medium humus or lowest (Sh) at medium humus (Fig. 6B). The only case where static and dynamic incubations gave approximately the same result at all three humus levels was deep mixing with high speed (Dh) in 2014, where the difference between static and dynamic incubations was, respectively, − 1.5%, + 5.8% and + 7.9% for the three humus levels (Fig. 6B).
Calculated depths where the production m−3 as given by the static incubations corresponded to the production given by the dynamic incubations in experiments April 2013 (A) and April 2014 (B). Integration 1 and 2 is the depth where the average production m−3 for 0–4.5 m (Integration 1) respectively 0–1.5 m (Integration 2) occurs. The six treatments Ss through Dh represent the dynamic incubations with shallow (S) or deep (D) mixing and slow (s), medium (m) or high (h) mixing speed. Stars in A indicate that the calculated depth is not covered by the integration equations
ANOVA tests (Table 4) showed significant (P < 0.05) effects on primary production from humus addition and mixing rate only for the deep mixing, 0–4.5 m, in April 2013. However, there was a strong interacting effect between humus and mixing in three of four cases, meaning that the effect of humus was strongly dependent of the mixing level.
Discussion
The rotating speed in my experiments ranged from 0.6 to 13.2 cm s−1, considerably faster than the deck incubator used by Gocke & Lenz (2004). The rotation speed is probably an important factor since slow mixing makes it possible for physiological adaptations to respond to the light fluctuations and the individual cells can continuously adjust their photosynthesis to the new conditions (Vincent, 1980; Falkowski & Wirick, 1981; Falkowski, 1983). With more intense mixing, the effect on photosynthesis is more uncertain, since the response times to adapt to low irradiance levels are usually shorter than those for adaptation to high irradiance levels (cf. review by Ferris & Christian, 1991). Model runs by Ross et al. (2011a) indicated that a low mixing time scale (high rotation speed) in their model gave more divergent results from static incubations than results from high mixing time scales. ANOVA results in my experiments showed statistically significant effects of mixing speed only for the deep mixing layer in April 2013, although the interacting effect with humus was statistically significant in three of the four experimental series (cf. Table 4).
If the primary production was directly controlled by the total PAR dose received during the incubations (see Table 2), we would expect a gradually diverging difference between shallow and deep dynamic incubations with increased humus, with the extreme of 33.3, respectively, 21.0 times higher primary production in shallow dynamic incubations and high humus in 2013, respectively, 2014. In reality, the primary production was 1.4 times higher in 2013 (Fig. 3E) and 2.7 times higher in 2014 (Fig. 4E). Thus, the PAR dose does not provide a guideline for predicting primary production for dynamic incubations. The same impression is given from the calculated standardised primary production estimates (Table 3), where especially the dynamic incubations in the deep mixed layer could compensate the much lower average irradiance experienced by humus addition, by a much higher photosynthetic efficiency.
My results indicated that the difference between static and dynamic incubations increased with increased PAR exposure gradient across the mixing layer (increased humus content), but that there was a strong interacting effect of mixing intensity (Figs. 3, 4, Table 4). The different outfall between the two years might also partly be a consequence of differences in the optical environment at the start situation (no humus added), where the phytoplankton community in April 2013 experienced a stronger reduction in the optical climate (see Figs. 3, 4). Another factor that might be of significance for explaining differences in results between the two experimental series is the difference in pre-adaptation to different euphotic depths. In 2013, humus was added both to the mesocosm tank where the water was collected and the incubation tank, whereas in 2014, humus was only added to the incubation tank. In 2013, therefore, the phytoplankton community was adapted to a mixing depth shallower than the euphotic depth with no humus, and gradually more shallow euphotic depth than the mixing depth with the two humus additions (cf Table 2). In 2014, the phytoplankton community used in the incubation tank was taken from a mixed 4.5 m water column where the euphotic depth was 3.6 m (Table 2) and this community was used in the incubation tank where the euphotic depth was gradually decreased with the two additions of humus (cf. Table 2). Thus, in 2014, the phytoplankton community was adapted to a higher irradiance range than used in the incubations with added humus, whereas in 2013, they were adapted to the same irradiance range as in the incubations. Since there are differences in the response times of physiological adaptations to changes in light, with some adaptations to low light levels being slower than to high light (Ferris & Christian, 1991), this methodological difference might explain much of the observed differences. However, simultaneous experiments with and without pre-conditioning would be needed to evaluate the significance of such treatment differences. There was also a strong difference in March/April between the 2 years in phytoplankton community structure in the field where the seawater supply originated (see Materials and methods) that probably was of significance. In 2013, there was a strong dominance of dinoflagellates, whereas in 2014, diatoms dominated strongly. Since different phytoplankton groups differ in their composition of light-absorbing pigments (Wright & Jeffrey, 1987; Latasa et al., 1992) and the spectral distribution changes towards higher wavelengths in humus-rich water (cf. Fig. 2), humus-rich water should thereby favour species that can use the longer PAR wavelengths more efficiently (Bidigare et al., 1990). Phytoplankton responses on vertical mixing might therefore differ between environments with humus-rich water and clear-water environments. However, I never analysed the actual phytoplankton composition in the experimental tanks, so this suggested explanation remains as a speculation.
The main aim of this study was to evaluate if field measurements of primary production could be simplified and improved to give higher accuracy regarding the “true production”. My experiments with vertically rotating incubations, simulating mixing by Lagmuir circulation, were intended to evaluate how mixing intensity and mixing depth would govern primary production in different light environments. Although the ANOVA results (Table 4) only indicated significant effects of humus on the primary production, it is well known that humus and mixing depth strongly influence primary production (e.g. Båmstedt & Wikner, 2016). The rather high variability in my data and the significant interacting effects thus weakened the conclusion taken from this test. Since it is difficult to decide which method best mirrors the true primary production in the natural environment, it is also difficult to evaluate if alternative methods increase the accuracy in the estimates. The results for the four single-point measurements (AD, LD, DAL, DLL) responded in the same way to added humus in 2013 (Fig. 5A, B), and usually gave relatively similar production estimates as static incubation series (Integration), with exception for shallow depth and no humus (Fig. 5B). However, the result for the deep mixed layer in 2014 showed consistently higher results for DAL and consistently lower results for AD, LD and DLL compared to Integration (Fig. 5C), whereas the shallow mixed layer showed more homogenous results for the four single-point estimates, and also a closer similarity with the integration estimates (Fig. 5D). If we consider “Integration” as the method that gives the true results, simplifying this by only using incubations at a single depth, determined either by the light profile or average depth of the mixed layer will therefore not give a high accuracy in estimating true water-column production. My results for dynamic incubations neither mirrored integration estimates (Fig. 5A–D, Integration) or single-point estimates (Fig. 5A–D, AD, LD, DAL, DLL). A similar picture is given by the estimated corresponding production depths (Fig. 6) where only high-speed mixing in the deep mixed layer (treatment Dh) in 2014 responded to humus addition in the same way as “Integration 1”, differing only between 1 and 8% (Fig. 6B).
Previous results from mathematical models of algal growth and productivity have indicated relatively small differences between static and dynamic incubations. Ross et al. (2011a) presented an individual-based combined turbulence and photosynthesis model that showed a potential divergence between in situ growth of algae (turbulent mixing applied) and in vitro growth (no turbulence), both when based on cell contents of chlorophyll and of carbon. Their theoretical approach should correspond to my two incubation methods, where the results from the dynamic incubations (rotated incubations) should mirror their in situ growth and the results from the static incubations corresponding to their in vitro growth. Turbulence intensity, optical depth of the mixing layer, i.e. k × H (k = light attenuation coefficient, H = depth of mixed layer) and incubation depth within the mixed layer were the factors that defined the degree of difference between the two model runs. Model results also showed that minimum difference between in situ and in vivo growth was close to or slightly above the mean depth of the mixed layer as long as the optical depth of the mixed layer was ≤ 6. Increased optical depth should move this point further up. If we subtract error ɛ2 from error ɛ1 in Ross et al. (2011a), we get an expression of the difference between carbon-based in situ growth rate, i.e. dynamic incubation, and carbon-based in vitro growth rate, i.e. static incubation, which, according to Fig. 7b in Ross et al. (2011b) should be negative down to just above the mean depth of the mixed layer, where static and dynamic incubations should be the same. In my experiments, midpoint depth of the mixed layer would be 2.25 m and 0.75 m for, respectively, deep and shallow mixed layer. The depth for average water-column production ranged from 1.17 to 1.46 m in 2013 and from 1.56 to 2.06 m in 2014 for the deep mixed layer, and between 0.73 and 1.02 m in 2013 and between 0.89 and 1.42 m in 2014 for the shallow mixed layer (see Fig. 6A, B, Integration1, Integration 2). Furthermore, dynamic incubations only occasionally gave the same calculated production depth as the static incubations (Fig. 6A, B), thus diverging from the proposed similarity for static and dynamic incubations and a mean water-column production close to midpoint mixed layer (Ross et al., 2011a, b).
Ross et al. (2011b) estimated the difference between water-column primary production with turbulent mixing versus that without mixing, using the model presented in Ross et al. (2011a). They concluded that for most environmental scenarios, the differences would be < 15%, with a maximum of 25% and a minimum of 2%. Sensitivity for changes in model settings was only observed for environments with high values of optical depth (k × H, see above) and a low mixing time scale (H2/Km, where H = mixed layer depth and Km = turbulent diffusivity at the mid depth of the mixed layer). From their model runs, they proposed that for a highly turbid mixed layer with a depth exceeding the euphotic depth by a factor 2 or more, the static incubations could overestimate the in situ productivity by up to 25%, and that a shorter incubation time than 24 h would reduce this error. My experimental results could not verify the model predictions of Ross et al. (2011b). With no humus addition, the shallow mixed layer had an optical depth of 1.4 and 1.9 in the two years, and primary production from dynamic incubations ranged between 33 and 62% of the static incubations (cf. Fig. 5A, C). In April 2013, the increased optical depth caused by humus addition changed the primary production towards higher ratios for dynamic/static incubations, where the most extreme difference was recorded for high humus and deep mixed layer, with an optical depth of 10.8, and a ratio of 9.5 (see Fig. 5A, label Dm).
Conclusions
Estimating planktonic primary production in a natural aquatic ecosystem is a difficult task, since a number of variable factors are of significant importance. Even if the production is primarily light controlled (no nutrient limitations), Lagmuir circulation and turbulent mixing cause irradiance variations for the phytoplankton cells that are not mirrored in static incubations. Since different species adapt differently to variations in the quantity (photons) and quality (spectral distribution) of the irradiance, the bulk result in primary production, which is the summed result of each species in relation to its proportional abundance, will differ from static incubations. Due to a slower adaptation to low irradiance levels than to high irradiance levels, a variable irradiance level, as generated by vertical mixing, will affect the photo-assimilation differently than at conditions during static incubations. The transparency (light attenuation) and wavelength-dependent absorption of the water and the mixing characteristics (mixing depth and mixing intensity) are factors of importance for the difference in primary production between static and dynamic incubations. Furthermore, recent pre-history of the community regarding the optical environment will influence the results from both static and dynamic incubations. Non-consistent results, including the present ones, do not provide clear guidelines for a “best practice” in primary production measurements. Estimating water-column primary production by using only a single incubation depth or a single irradiance level, defined by the in situ light profile or depth of the mixed layer, will cause inaccurate results and cannot be recommended. The present results show that even recent mathematical models cannot accurately predict the primary production in a natural and bio-physically complex ecosystem.
References
Båmstedt, U. & H. Larsson, 2018. An indoor pelagic mesocosm facility to simulate multiple water-column characteristics. International Aquatic Research 10: 13–29.
Båmstedt, U. & J. Wikner, 2016. Mixing depth and allochthonous dissolved organic carbon: controlling factors of coastal trophic balance. Marine Ecology Progress Series 561: 17–29.
Bertoni, R. & E. Balseiro, 2005. Mixing layer running incubator (MIRI): an instrument for incubating samples while moving vertically in the mixing layer. Limnology and Oceanography-Methods 3: 158–163.
Bertoni, R., W. H. Jeffrey, M. Pujo-Pay, L. Oriol, P. Conan & F. Joux, 2011. Influence of water mixing on the inhibitory effect of UV radiation on primary and bacterial production in Mediterranean coastal water. Aquatic Sciences 73: 377–387.
Bidigare, R. R., J. Marra, T. D. Dickey, R. Iturriaga, K. S. Baker, R. C. Smith & H. Pak, 1990. Evidence for phytoplankton succession and chromatic adaptation in the Sargasso Sea during spring 1985. Ecology Progress Series 60: 113–122.
Colijn, F., L. Edler (eds), 1998. Working manual and supporting papers on the use of a standardised incubator—technique in primary production measurements. In: Report of the working group on phytoplankton ecology. CM 1998/C: 3. ICES,Copenhagen, pp 49–57
Delgadillo-Hinojosa, F., G. Gaxiola-Castro, J. A. Segovia-Zavala, A. Munoz-Barbosa & M. V. Orozco-Borbon, 1997. The effect of vertical mixing on primary production in a bay of the Gulf of California. Estuarine Coastal and Shelf Science 45: 135–148.
Denman, K. L. & A. E. Gargett, 1983. Time and space scales of vertical mixing and advection of phytoplankton in the upper ocean. Limnology and Oceanography 28: 801–815.
Edwards, K. F., M. K. Thomas, C. A. Klausmeier & E. Litchman, 2016. Phytoplankton growth and the interaction of light and temperature: a synthesis at the species and community level. Limnology and Oceanography 61: 1232–1244.
Falkowski, P. G., 1983. Light-shade adaptation and vertical mixing of marine phytoplankton—a comparative field study. Journal of Marine Research 41: 215–237.
Falkowski, P. G. & C. D. Wirick, 1981. A simulation-model of the effects of vertical mixing on primary productivity. Marine Biology 65: 69–75.
Farmer, D. & C. Mcneil, 1999. Photoadaptation in a convective layer. Deep-Sea Research Part Ii-Topical Studies in Oceanography 46: 2433–2446.
Ferris, J. M. & R. Christian, 1991. Aquatic primary production in relation to microalgal responses to changing light—a review. Aquatic Sciences 53: 187–217.
Gali, M., R. Simo, G. L. Perez, C. Ruiz-Gonzalez, H. Sarmento, S. J. Royer, A. Fuentes-Lema & J. M. Gasol, 2013. Differential response of planktonic primary, bacterial, and dimethylsulfide production rates to static vs. dynamic light exposure in upper mixed-layer summer sea waters. Biogeosciences 10: 7983–7998.
Gallegos, C. L. & T. Platt, 1982. Phytoplankton production and water motion in surface mixed layers. Deep-Sea Research Part a-Oceanographic Research Papers 29: 65–76.
Gallegos, C. L. & T. Platt, 1985. Vertical advection of phytoplankton and productivity estimates—a dimensional analysis. Marine Ecology Progress Series 26: 125–134.
Gargas, E., 1975. A manual for phytoplankton primary production studies in the Baltic. Baltic Marine Biologists and the Danish Agency of Environmental Protection, København.
Gocke, K. & J. Lenz, 2004. A new ‘turbulence incubator’ for measuring primary production in non-stratified waters. Journal of Plankton Research 26: 357–369.
Grobbelaar, J. U., 1989. The contribution of phytoplankton productivity in turbid fresh-waters to their trophic status. Hydrobiologia 173: 127–133.
Havelkova-Dousova, H., O. Prasil & M. J. Behrenfeld, 2004. Photoacclimation of Dunaliella tertiolecta (Chlorophyceae) under fluctuating irradiance. Photosynthetica 42: 273–281.
Helbling, E. W., P. Carrillo, J. M. Medina-Sanchez, C. Duran, G. Herrera, M. Villar-Argaiz & V. E. Villafane, 2013. Interactive effects of vertical mixing, nutrients and ultraviolet radiation: in situ photosynthetic responses of phytoplankton from high mountain lakes in Southern Europe. Biogeosciences 10: 1037–1050.
Helbling, E. W., K. S. Gao, R. J. Goncalves, H. Y. Wu & V. E. Villafane, 2003. Utilization of solar UV radiation by coastal phytoplankton assemblages off SE China when exposed to fast mixing. Marine Ecology Progress Series 259: 59–66.
Joiris, C. & A. Bertels, 1985. Incubation under fluctuating light conditions provides values much closer to real insitu primary production. Bulletin of Marine Science 37: 620–625.
Kirk, J. T. O., 1994. Light and Photosynthesis in Aquatic Ecosystems, 2nd ed. Cambridge University Press, Cambridge: 509.
Köhler, J., 1997. Measurement of in situ growth rates of phytoplankton under conditions of simulated turbulence. Journal of Plankton Research 19: 849–862.
Kromkamp, J. & M. Limbeek, 1993. Effect of short-term variation in irradiance on light-harvesting and photosynthesis of the marine diatom Skeletonema costatum—a laboratory study simulating vertical mixing. Journal of General Microbiology 139: 2277–2284.
Latasa, M., M. Estrada & M. Delgado, 1992. Plankton-pigment relationships in the northwestern Mediterranean during stratification. Marine Ecology Progress Series 88: 61–73.
Lawrenz, E. & T. L. Richardson, 2017. Differential effects of changes in spectral irradiance on photoacclimation, primary productivity and growth in Rhodomonas salina (Cryptophyceae) and Skeletonema costatum (Bacillariophyceae) in simulated blackwater environments. Journal of Phycology 53: 1241–1254.
Lizon, F., L. Seuront & Y. Lagadeuc, 1998. Photoadaptation and primary production study in tidally mixed coastal waters using a Lagrangian model. Marine Ecology Progress Series 169: 43–54.
Marra, J., 1978a. Effect of short-term variations in light-intensity on photosynthesis of a marine phytoplankter—laboratory simulation study. Marine Biology 46: 191–202.
Marra, J., 1978b. Phytoplankton photosynthetic response to vertical movement in a mixed layer. Marine Biology 46: 203–208.
Patterson, J. C., 1991. Modelling the effect of motion on primary production in the mixed layer of lakes. Aquatic Sciences 53: 218–238.
Randall, J. M. & J. W. Day, 1987. Effect of river discharge and vertical circulation on aquatic primary production in a turbid Louisiana (USA) estuary. Netherlands Journal of Sea Research 21: 231–242.
Ross, O. N., R. J. Geider, E. Berdalet, M. L. Artigas & J. Piera, 2011a. Modelling the effect of vertical mixing on bottle incubations for determining in situ phytoplankton dynamics. I. Growth rates. Marine Ecology Progress Series 435: 13–31.
Ross, O. N., R. J. Geider & J. Piera, 2011b. Modelling the effect of vertical mixing on bottle incubations for determining in situ phytoplankton dynamics. II. Primary production. Marine Ecology Progress Series 435: 33–45.
Tirok, K. & U. Gaedke, 2007. The effect of irradiance, vertical mixing and temperature on spring phytoplankton dynamics under climate change: long-term observations and model analysis. Oecologia 150: 625–642.
Vincent, W. F., 1980. Mechanisms of rapid photosynthetic adaptation in natural phytoplankton communities. 2. Changes in photochemical capacity as measured by DCMU-induced chlorophyll fluorescence. Journal of Phycology 16: 568–577.
Walsh, P. & L. Legendre, 1983. Photosynthesis of natural phytoplankton under high-frequency light fluctuations simulating those induced by sea-surface waves. Limnology and Oceanography 28: 688–697.
Wright, S. W. & S. W. Jeffrey, 1987. Fucoxanthin pigment markers of marine phytoplankton analyzed by HPLC and HPTLC. Marine Ecology Progress Series 38: 259–266.
Yoder, J. A. & S. S. Bishop, 1985. Effects of mixing-induced irradiance fluctuations on photosynthesis of natural assamblages of coastal phytoplankton. Marine Biology 90: 87–93.
Acknowledgements
Thanks are due to Umeå Marine Sciences Centre, university of Umeå, for providing excellent working facilities. Mikael Molin, Jonas Wester and Tommy Olofsson helped in constructing the apparatus for rotating incubations and Henrik Larsson provided valuable help when starting up the mesocosm tanks. Information on the phytoplankton community were taken from the Swedish environmental monitoring program with the database dBothnia at Umeå Marine Sciences Centre, which is thankfully appreciated. Comments from two anonymous referees helped improving the manuscript considerably and are highly appreciated. This project was part of the Strategic Marine Environmental Research program “Ecosystem dynamics in the Baltic Sea in a changing climate perspective” (ECOCHANGE).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Lacopo Bertocci
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Båmstedt, U. Comparing static and dynamic incubations in primary production measurements under different euphotic and mixing depths. Hydrobiologia 827, 155–169 (2019). https://doi.org/10.1007/s10750-018-3762-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-018-3762-1