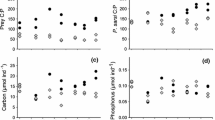
Abstract
Cladocerans feed on a variety of phytoplankton food sources, which are variable across space and time. Different phytoplankton groups represent different nutritional quality to Daphnia magna (Straus) due to differences in their Redfield stoichiometry or digestibility. We used chlorophyll fluorescence to directly measure phytoplankton group quantity and composition (green algae and cyanobacteria) in the guts of live D. magna and thereby directly assessed differences in gut transit time (GTT). We observed a decrease of GTT with lower food quality relative to the most suitable diet treatment composed only of the green alga: Scenedesmus obliquus (Turpin) Kützing (GTT = 27 min 20 s). Mixed, lower quality diets composed of 75% cyanobacteria, Microcystis aeruginosa (Kützing) and 25% S. obliquus resulted in faster GTT, as did diets exclusively composed of microcystin-lacking cyanobacteria (GTT = 19 min 42 s). The GTT could not be measured when diets were composed exclusively of microcystin-producing M. aeruginosa due to insignificant ingestion of the cyanobacteria. Using Ivlev’s Electivity index, we demonstrate that provided with mixed algal food, D. magna was able to avoid ingesting lower quality food (cyanobacteria) in favor of more valuable material (green alga). However, this ability decreases or is lost when exposed to food mixtures dominated by cyanobacteria.




Similar content being viewed by others
References
Alva-Martínez, A. F., S. S. S. Sarma & S. Nandini, 2004. Population growth of Daphnia pulex (Cladocera) on a mixed diet (Microcystis aeruginosa with Chlorella or Scenedesmus). Crustaceana 77: 973–988.
Baxter, L. R., P. K. Sibley, K. R. Solomon & M. L. Hanson, 2013. Interactions between atrazine and phosphorus in aquatic systems: effects on phytoplankton and periphyton. Chemosphere 90: 1069–1076.
Burns, C. W., 1969. Relation between filtering rate, temperature, and body size in four species of Daphnia. Limnology and Oceanography 14: 693–700.
Caparroy, P. & F. Carlotti, 1996. A model for Acartia tonsa: effect of turbulence and consequences for the related physiological processes. Journal of Plankton Research 18: 2139–2177.
Chen, W., L. Song, D. Ou & N. Gan, 2005. Chronic toxicity and responses of several important enzymes in Daphnia magna on exposure to sublethal microcystin-LR. Environmental Toxicology 20: 323–330.
Coelho, H., R. Calado, A. O. Olaguer-Feliú, S. Vieira, H. Queiroga & J. Serôdio, 2009. Nondestructive quantification of phytoplankton gut content of brachyuran crab megalopae using in vivo chlorophyll a fluorescence. Journal of Plankton Research 31: 577–581.
DeMott, W. R. & D. C. Müller-Navarra, 1997. The importance of highly unsaturated fatty acids in zooplankton nutrition: evidence from experiments with Daphnia, a cyanobacterium and lipid emulsions. Freshwater Biology 38: 649–664.
DeMott, W. R., Z. Qing-Xue & W. W. Carmichael, 1991. Effects of toxic cyanobacteria and purified toxins on the survival and feeding of a copepod and three species of Daphnia. Limnology & Oceanography 36: 1346–1357.
Downing, J. A. & F. H. Rigler, 1984. A manual on methods for the assessment of secondary production in fresh waters, IBP Handbook No. 17, 2nd ed. Blackwell Science Publisher, Oxford: 500.
Ferrão-Filho, A. S., S. M. F. O. Azevedo & W. R. DeMott, 2000. Effects of toxic and non-toxic cyanobacteria on the life history of tropical and temperate cladocerans. Freshwater Biology 45: 1–19.
Ghadouani, A., B. Pinel-Alloul, K. Plath, G. A. Codd & W. Lampert, 2004. Effects of Microcystis aeruginosa and purified microcystin-LR on the feeding behavior of Daphnia pulicaria. Limnology and Oceanography 49: 666–679.
Gulati, R. D. & W. R. DeMott, 1997. The role of food quality for zooplankton: remarks on the state-of-the-art, perspectives and priorities. Freshwater Biology 38: 753–768.
Guo, N. & P. Xie, 2006. Development of tolerance against toxic Microcystis aeruginosa in three cladocerans and the ecological implications. Environmental Pollution 143: 513–518.
Haney, J. F., 1985. Regulation of cladoceran filtering rates in nature by body size, food concentration, and diel feeding patterns. Limnology and Oceanography 30: 397–411.
Hansen, B., P. K. Bjornsen & P. J. Hansen, 1994. The size ratio between planktonic predators and their prey. Limnology and Oceanography 39: 395–403.
Hecky, R. E. & P. Kilham, 1988. Nutrient limitation of phytoplankton in freshwater and marine environments: a review of recent evidence on the effects of enrichment. Limnology and Oceanography 33: 796–822.
Kilham, S. S., D. A. Kreeger, S. G. Lynn, C. E. Goulden & L. Herrera, 1998. COMBO: a defined freshwater culture medium for algae and zooplankton. Hydrobiologia 377: 147–159.
Lampert, W., 1987. Laboratory studies on zooplankton-cyanobacteria interactions. New Zealand Journal of Marine & Freshwater Research 21: 483–490.
Lechowicz, M. J., 1982. The sampling characteristics of electivity indices. Oecologia 52: 22–30.
Lürling, M., 2003a. Effects of microcystin-free and microcystin-containing strains of the cyanobacterium Microcystis aeruginosa on growth of the grazer Daphnia magna. Environmental Toxicology 18: 202–210.
Lürling, M., 2003b. Daphnia growth on microcystin-producing and microcystin-free Microcystis aeruginosa in different mixtures with the green alga Scenedesmus obliquus. Limnology and Oceanography 48: 2214–2220.
Lürling, M. & E. van der Grinten, 2003. Life-history characteristics of Daphnia exposed to dissolved microcystin-LR and to the cyanobacterium Microcystis aeruginosa with and without microcystins. Environmental Toxicology and Chemistry 22: 1281–1287.
Lürling, M. & E. van Donk, 1997. Life history consequences for Daphnia pulex feeding on nutrient-limited phytoplankton. Freshwater Biology 38: 693–709.
Murtaugh, P. A., 1985. The influence of food concentration and feeding rate on the gut residence time of Daphnia. Journal of Plankton Research 7: 415–420.
Müller-Navarra, D., 1995. Evidence that a highly unsaturated fatty acid limits Daphnia growth in nature. Archiv für Hydrobiologie 132: 297–307.
Nizan, S., C. Dimentman & M. Shilo, 1986. Acute toxic effects of the cyanobacterium Microcystis aeruginosa on Daphnia magna. Limnology and Oceanography 31: 497–502.
Nogueira, I. C. G., M. L. Saker, S. Pflugmacher, C. Wiegand & V. M. Vasconcelos, 2004. Toxicity of the cyanobacterium Cylindrospermopsis raciborskii to Daphnia magna. Environmental Toxicology 19: 453–459.
Pérez-Morales, A., S. S. S. Sarma & S. Nandini, 2014. Feeding and filtration rates of zooplankton (rotifers and cladocerans) fed toxic cyanobacterium (Microcystis aeruginosa). Journal of Environmental Biology 35: 1013–1020.
Peters, R. H. & J. A. Downing, 1984. Empirical analysis of zooplankton filtering and feeding rates. Limnology and Oceanography 29: 763–784.
Porter, K. G., 1973. Selective grazing and differential digestion of algae by zooplankton. Nature 244: 179–180.
Rhee, G.-Y. & I. J. Gotham, 1980. Optimum N: P ratios and coexistence of planktonic algae. Journal of Phycology 16: 486–489.
Richardson, K., J. Beardall & J. A. Raven, 1983. Adaptation of unicellular algae to irradiance: an analysis of strategies. New Phytologist 93: 157–191.
Rohrlack, T., E. Dittmann, M. Henning, T. Börner & J. G. Kohl, 1999a. Role of microcystins in poisoning and food ingestion inhibition of Daphnia galeata caused by the cyanobacterium Microcystis aeruginosa. Applied and Environmental Microbiology 65: 737–739.
Rohrlack, T., M. Henning & J. G. Kohl, 1999b. Mechanisms of the inhibitory effect of the cyanobacterium Microcystis aeruginosa on Daphnia galeata’s ingestion rate. Journal of Plankton Research 21: 1489–1500.
Rohrlack, T., E. Dittmann, T. Börner & K. Christoffersen, 2001. Effects of cell-bound microcystins on survival and feeding of Daphnia spp. Applied and Environmental Microbiology 67: 3523–3529.
Sarma, S. S. S. & S. Nandini, 2006. Review of recent ecotoxicological studies on cladocerans. Journal of Environmental Science and Health – Part B Pesticides, Food Contaminants, and Agricultural Wastes 41: 1417–1430.
Sastri, A. R., B. E. Beisner & P. Juneau, 2011. In vivo determination of Daphnia feeding rates using PAM fluorometry. Journal of Plankton Research 33: 1455–1459.
Seidendorf, B., N. Meier, A. Petrusek, M. Boersma, B. Streit & K. Schwenk, 2010. Sensitivity of Daphnia species to phosphorus-deficient diets. Oecologia 162: 349–357.
Shapiro, J., 1973. Blue-green algae: why they become dominant. Science 179: 382–384.
Taipale, S. J., M. J. Kainz & M. T. Brett, 2011. Diet-switching experiments show rapid accumulation and preferential retention of highly unsaturated fatty acids in Daphnia. Oikos 120: 1674–1682.
van Donk, E. & D. O. Hessen, 1993. Grazing resistance in nutrient-stressed phytoplankton. Oecologia 93: 508–511.
Weber C. I., 1993. Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. 4th ed. United States Environmental Protection Agency, Cincinnati, Ohio, EPA/600/4-90/027F, xv + 293 p.
Acknowledgements
This research was supported by the Natural Science and Engineering Research Council of Canada (NSERC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: S. Nandini, S.S.S. Sarma, Erik Jeppesen & Linda May / Shallow Lakes Research: Advances and Perspectives
Rights and permissions
About this article
Cite this article
Chesney, T., Sastri, A.R., Beisner, B.E. et al. Application of fluorometry (Phyto-PAM) for assessing food selection by cladocerans. Hydrobiologia 829, 133–142 (2019). https://doi.org/10.1007/s10750-018-3753-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-018-3753-2