Abstract
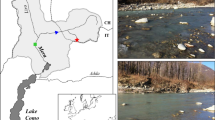
Development of effective conservation and restoration strategies for freshwater pearly mussels requires identification of environmental constraints on the distributions of individual mussel species. We examined whether the spatial distribution of the endangered Alasmidonta heterodon in Flat Brook, a tributary of the upper Delaware River, was constrained by water chemistry (i.e., calcium availability), bed mobility, or both. Alasmidonta heterodon populations were bracketed between upstream reaches that were under-saturated with respect to aragonite and downstream reaches that were saturated for aragonite during summer baseflow but had steep channels with high bed mobility. Variability in bed mobility and water chemistry along the length of Flat Brook create a “habitat window” for A. heterodon defined by bed stability (mobility index ≤1) and aragonite saturation (saturation index ≥1). We suggest the species may exist in a narrow biogeochemical window that is seasonally near saturation. Alasmidonta heterodon populations may be susceptible to climate change or anthropogenic disturbances that increase discharge, decrease groundwater inflow or chemistry, and thus affect either bed mobility or aragonite saturation. Identifying the biogeochemical microhabitats and requirements of individual mussel species and incorporating this knowledge into management decisions should enhance the conservation and restoration of endangered mussel species.




Similar content being viewed by others
References
Albert, R. & R. L. Limbeck, 2000. High flow management objectives for New Jersey non-coastal waters. Delaware River Basin Commission, West Trenton, NJ.
Allen, D. C. & C. C. Vaughn, 2010. Complex hydraulic and substrate variables limit freshwater mussel species richness and abundance. Journal of the North American Benthological Society 29: 383–394.
Ashton, M. J., 2010. Freshwater mussel records collected by the Maryland Department of Natural Resources’ Monitoring and Non-tidal Assessment Division (1995-2009): investigating environmental conditions and potential host fish of select species. Publication# 12-3112010-443. Annapolis, MD: Maryland Department of Natural Resources.
Bailey, R. C. & R. H. Green, 1988. Within-basin variation in shell morpphology and growth rate of a freshwater mussel. Canadian Journal of Zoology 66: 1704–1708.
Bauer, G., 1992. Variation in the life span and size of the freshwater pearl mussel. Journal of Animal Ecology 61: 425–436.
Bogan, A. E., 1993. Freshwater bivalve extinctions (Mollusca: Unionoida): a search for causes. American Zoologist 33: 599–609.
Bogan, A., 2008. Global diversity of freshwater mussels (Mollusca, Bivalvia) in freshwater. Hydrobiologia 595: 139–147.
Boxall, G. D., G. R. Giannico & H. W. Li, 2008. Landscape topography and the distribution of Lahontan cutthroat trout (Oncorhynchus clarki henshawi) in a high desert stream. Environmental Biology of Fishes 82: 71–84.
Candy, I., M. Stephens, J. Hancock & R. Waghorne, 2011. Palaeoenvironments of ancient humans in Britain: the application of oxygen and carbon isotopes to the reconstruction of Pleistocene environments. In Ashton, N. M., S. G. Lewis & C. B. Stringer (eds), The ancient human occupation of Britain. Developments in quaternary science, Vol. 14. Elsevier, Amsterdam: 23–37.
Cavalli, M., P. Tarolli, M. Lorenzo & G. Dalla Fontana, 2008. The effectiveness of airborne LiDAR data in the recognition of channel-bed morphology. Catena 73: 249–260.
Clancy K., & K. Prestegaard, 2006. Quantifying particle organization in boulder bed streams. In Parker, G. & M. H. Garcia (eds), River, Coastal, and Estuarine Morphodynamics: RCEM 2005. Taylor & Francis Group, plc, London: 71–77
DuBoys, M. P., 1879. Le Rhone et les rivieres a lit affouillable. Annales de Pontset Chausses 18(sec.5): 141–195.
French, S. K. & J. D. Ackerman, 2014. Responses of newly settled juvenile mussels to bed shear stress: implications for dispersal. Freshwater Science 33: 46–55.
Gangloff, M. M. & J. W. Feminella, 2007. Stream channel geomorphology influences mussel abundance in southern Appalachian streams, U.S.A. Freshwater Biology 52: 64–74.
Goewert, A., D. Surge, S. J. Carpenter & J. Downing, 2007. Oxygen and carbon isotope ratios of Lampsilis cardium (Unionidae) from two streams in agricultural watersheds of Iowa, USA. Palaeogeography, Palaeoclimatology, Palaeoecology 252: 637–648.
Gordon, N. D., T. A. McMahon, B. L. Finlayson, C. J. Gippel & R. J. Nathan, 2004. Stream hydrology: an introduction for ecologists. John Wiley & Sons Ltd, West Sussex.
Green, M. A., M. E. Jones, C. L. Boudreau, R. L. Moore & B. A. Westman, 2004. Dissolution mortality of juvenile bivalves in coastal marine deposits. Limnology and Oceanography 49: 727–734.
Green, M. A., G. G. Waldbusser, S. L. Reilly, K. Emerson & S. O’Donnell, 2009. Death by dissolution: sediment saturation state as a mortality factor for juvenile bivalves. Limnology and Oceanography 54: 1037–1047.
Green, M. A., G. G. Waldbusser, L. Hubazc, E. Cathcart & J. Hall, 2013. Carbonate mineral saturation state as the recruitment cue for settling bivalves in marine muds. Estuaries and Coasts 36: 18–27.
Haag, W. R., 2012. North American freshwater mussels: natural history, ecology, and conservation. Cambridge University Press, Cambridge.
Haag, W. R. & A. M. Commens-Carson, 2008. Testing the assumption of annual shell ring deposition in freshwater mussels. Canadian Journal of Fisheries and Aquatic Sciences 65: 493–508.
Hardison, B. S. & J. B. Layzer, 2001. Relations between complex hydraulics and the localized distribution of mussels in three regulated rivers. Regulated Rivers: Research & Management 17: 77–84.
Hauer, C., G. Mandlburger & H. Habersack, 2009. Hydraulically related hydro-morphological units: description based on a new conceptual mesohabitat evaluation model (MEM) using LiDAR data as geometric input. River Research and Applications 25: 29–47.
Hornbach, D. J., V. J. Kurth & M. C. Hove, 2010. Variation in freshwater mussel shell sculpture and shape along a river gradient. The American Midland Naturalist 164: 22–36.
Howard, J. K. & K. M. Cuffey, 2003. Freshwater mussels in a California North Coast Range river: occurrence, distribution, and controls. Journal of the North American Benthological Society 22: 63–77.
Kesler, D. H., T. J. Newton & L. Green, 2007. Long-term monitoring of growth in the Eastern Elliptio, Elliptio complanata (Bivalvia: Unionidae), in Rhode Island: a transplant experiment. Journal of the North American Benthological Society 26: 123–133.
Kurihara, H., T. Asai, S. Kato & A. Ishimatsu, 2009. Effects of elevated pCO2 on early development in the mussel Mytilus galloprovincialis. Aquatic Biology 4: 225–233.
Layzer, J. B. & L. M. Madison, 1995. Microhabitat use by freshwater mussels and recommendations for determining their instream flow needs. Regulated Rivers: Research & Management 10: 329–345.
Lefsky, M. A., W. B. Cohen, G. G. Parker & D. J. Harding, 2002. Lidar remote sensing for ecosystem studies. BioScience 52: 19–30.
Lellis, W. A., B. S. White, J. C. Cole, C. S. Johnson, J. L. Devers, E. V. S. Gray & H. S. Galbraith, 2013. Newly documented host fishes for the eastern elliptio mussel Elliptio complanata. Journal of Fish and Wildlife Management 4: 75–85.
Lenzi, M. A., L. Mao & F. Comiti, 2006. When does bedload transport begin in steep boulder-bed streams? Hydrological Processes 20: 3517–3533.
Leopold, L. B., M. G. Wolman & J. P. Miller, 1964. Fluvial processes in geomorphology. W.H. Freeman Company, San Francisco.
Locke, A., J. M. Hanson, G. J. Klassen, S. M. Richardson & C. I. Aube, 2003. The damming of the Petitcodiac River: species, populations, and habitats lost. Northeastern Naturalist 10: 39–54.
Lydeard, C., R. H. Cowie, W. F. Ponder, A. E. Bogan, P. Bouchet, S. A. Clark, K. S. Cummings, T. J. Frest, O. Gargominy, D. G. Herbert, R. Hershler, K. E. Perez, B. Roth, M. Seddon, E. E. Strong & F. G. Thompson, 2004. The global decline of nonmarine mollusks. BioScience 54: 321–330.
Mackie, G. L. & L. A. Flippance, 1983. Intra- and interspecific variations in calcium content of freshwater mollusca in relation to calcium content of the water. Journal of Molluscan Studies 49: 204–212.
Maloney, K. O., W. A. Lellis, R. M. Bennett & T. J. Waddle, 2012. Habitat persistence for sedentary organisms in managed rivers: the case for the federally endangered dwarf wedgemussel (Alasmidonta heterodon) in the Delaware River. Freshwater Biology 57: 1315–1327.
Marin, F., G. Luquet, B. Marie & D. Medakovic, 2007. Molluscan shell proteins: primary structure, origin, and evolution. Current Topics in Developmental Biology 80: 209–276.
McRae, S. E., J. D. Allan & J. B. Burch, 2004. Reach and catchment-scale determinants of the distribution of freshwater mussels (Bivalvia: Unionidae) in south-eastern Michigan, USA. Freshwater Biology 49: 127–142.
Michaelson, D. L. & R. J. Neves, 1995. Life history and habitat of the endangered dwarf wedgemussel Alasmidonta heterodon (Bivalvia: Unionidae). Journal of the North American Benthological Society 14: 324–340.
Morales, Y., L. J. Weber, A. E. Mynett & T. J. Newton, 2006. Effects of substrate and hydrodynamic conditions on the formation of mussel beds in a large river. Journal of the North American Benthological Society 25: 664–676.
Parker, B., 1978. Self-formed straight rivers with equilibrium banks and mobile bed. Part 2. The gravel river. Journal of Fluid Mechanics 89: 127–146.
Poff, N. L., J. D. Allan, M. B. Bain, J. R. Karr, K. L. Prestegaard, B. D. Richter, R. E. Sparks & J. C. Stromberg, 1997. The natural flow regime. BioScience 47: 769–784.
Prestegaard, K. L., 1983. Variables influencing water-surface slopes in gravel-bed streams at bankfull stage. Geological Society of America Bulletin 94: 673–678.
Pynnönen, K., 1991. Accumulation of 45Ca in the freshwater unionids Anodonta anatina and Unio tumidus, as influenced by water hardness, protons, and aluminum. The Journal of Experimental Zoology 260: 18–27.
Randhir, T. O. & A. G. Hawes, 2009. Watershed land use and aquatic ecosystem response: ecohydrologic approach to conservation policy. Journal of Hydrology 364: 182–199.
Rypel, A. L., W. R. Haag & R. H. Findlay, 2008. Validation of annual growth rings in freshwater mussel shells using cross dating. Canadian Journal of Fisheries and Aquatic Sciences 65: 2224–2232.
Salisbury, R. D., H. B. Kummel, C. E. Peet & G. N. Knapp, 1902. The glacial geology of New Jersey. MacCrellish & Quigley, Trenton, N.J.
Shaver, R. B., 1993. Field vs. lab alkalinity and pH: effects on ion balance and calcite saturation index. Groundwater Monitoring & Remediation 13: 104–112.
Shields, A., 1936. Application of the theory of similarity and turbulence research to the bedload movement. Transl.QMSaleh. Mitt.Preuss.Vers.WasserbauSchiffbau, 26th, Berlin.
Smith, D. G., 1985. Recent range expansion of the freshwater mussel Anodonta implicata and its relationship to clupeid fish restoration in the Connecticut River system. Freshwater Invertebrate Biology 4: 105–108.
Spooner, D. E. & C. C. Vaughn, 2006. Context-dependent effects of freshwater mussels on stream benthic communities. Freshwater Biology 51: 1016–1024.
Steuer, J. J., T. J. Newton & S. J. Zigler, 2008. Use of complext hydraulic variables to predict the distribution and density of unionids in a side channel of the Upper Mississippi River. Hydrobiologia 610: 67–82.
Strayer, D. L., 1993. Macrohabitats of freshwater mussels (Bivalvia:Unionacea) in streams of the north Atlantic Slope. Journal of the North American Benthological Society 12: 236–246.
Strayer, D. L., 1999. Use of flow refuges by unionid mussels in rivers. Journal of the North American Benthological Society 18: 468–476.
Strayer, D. L., 2008. Freshwater mussel ecology: a multifactor approach to distribution and abundance. University of California Press, Los Angeles.
Strayer, D. L. & J. Ralley, 1993. Microhabitat use by an assemblage of stream-dwelling unionaceans (Bivalvia), including two rare species of Alasmidonta. Journal of the North American Benthological Society 12: 247–258.
Strayer, D. L., S. J. Sprague & S. Claypool, 1996. A range-wide assessment of populations of Alasmidonta heterodon, an endangered freshwater mussel (Bivalvia:Unionidae). Journal of the North American Benthological Society 15: 308–317.
Strayer, D. L., J. A. Downing, W. R. Haag, T. L. King, J. B. Layzer, T. J. Newton & S. J. Nichols, 2004. Changing perspectives on pearly mussels, North America’s most imperiled animals. BioScience 54: 429–439.
Stumm, W. & J. Morgan, 1981. Aquatic chemistry: an introduction emphasizing chemical equilibria in natural waters. John Wiley & Sons, New York.
Thoma, D. P., S. C. Gupta, M. E. Bauer & C. E. Kirchoff, 2005. Airborne laser scanning for riverbank erosion assessment. Remote Sensing of Environment 95: 493–501.
Tóth, J., 1963. A theoretical analysis of groundwater flow in small drainage basins. Journal of Geophysical Research 68: 4795–4812.
Tóth, J., 1970. A conceptual model of the groundwater regime and the hydrogeologic environment. Journal of Hydrology 10: 164–176.
U.S. Environmental Protection Agency, 2011. A field-based aquatic life benchmark for conductivity in central Appalachian streams (final report). EPA/600/R-10/023F, U.S. Environmental Protection Agency, Washington, D.C.
U.S. Fish and Wildlife Service, 1993. Dwarf wedge mussel (Alasmidonta heterodon) recovery plan. U.S. Fish and Wildlife Service, Northeast Region, Hadley, MA.
U.S. Fish and Wildlife Service, 2007. Dwarf wedgemussel (Alasmidonta heterodon) 5-year review: summary and evaluation. U.S. Fish and Wildlife Service, Concord, NH.
Vaughn, C., K. Gido & D. Spooner, 2004. Ecosystem processes performed by unionid mussels in stream mesocosms: species roles and effects of abundance. Hydrobiologia 527: 35–47.
Wang, N., C. G. Ingersoll, D. K. Hardesty, C. D. Ivey, J. L. Kunz, T. W. May, F. J. Dwyer, A. D. Roberts, T. Augspurger, C. M. Kane, R. J. Neves & M. C. Barnhart, 2007. Acute toxicity of copper, ammonia, and chlorine to glochidia and juveniles of freshwater mussels (Unionidae). Environmental Toxicology and Chemistry 26: 2036–2047.
Wilbur, K. M., 1964. Shell formation and regeneration. In Wilbur, K. M. & C. M. Yonge (eds), Physiology of Mollusca. Academic Press, New York: 243–282.
Wolman, M. G., 1954. A method of sampling coarse river-bed material. Transactions of the American Geophysical Union 35: 951–956.
Acknowledgments
The authors thank Will Condon for assistance with field work. Comments by Teresa Newton, Andrew Elmore, Dale Honeyfield, Dan Spooner, and three anonymous reviewers improved the manuscript. Work was conducted under National Park Service Scientific Research and Collecting Permit number DEWA-2010-SCI-0017 and New Jersey Scientific Collecting Permit number SC 2010127. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Sonja Stendera
Rights and permissions
About this article
Cite this article
Campbell, C.A., Prestegaard, K.L. Physical and chemical constraints limit the habitat window for an endangered mussel. Hydrobiologia 772, 77–91 (2016). https://doi.org/10.1007/s10750-016-2642-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2642-9