Abstract
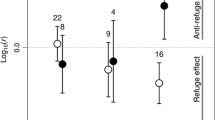
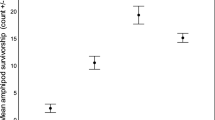
The ability to respond to a predation threat may be the key factor influencing prey survival. Thus, small-sized fish may adapt to use macrophyte patches as refugia in ecosystems where they face predators. We evaluated the habitat choices of a small fish species (Serrapinnus notomelas) to determine whether these fish prefer native versus recently introduced submerged macrophyte stands in the context of predator avoidance. Specifically, we applied three predator cue treatments: no cue, chemical cue from a hungry predator and presence of a satiated predator. First, we empirically tested the theoretical assumption that the prey fish use vegetated habitats and that the presence of an actual predator has a stronger effect on the choice of habitat than simply a chemical cue. Then we tested the hypothesis that prey do not choose a habitat according to macrophyte species and whether this pattern changed as a result of increasing predation risk. We found that the prey fish preferred vegetated habitats; however, they did not appear to distinguish native from invasive macrophytes. Our results support the hypothesis that the physical structure of macrophytes is more important in determining habitat choice than the evolutionary relationship between the fish and the native macrophyte species.


Similar content being viewed by others
References
Abjornsson, K., B. Wagner & A. Axelsson, 1997. Responses to Acilius sulcatus (Coleoptera: Dytiscidae) to chemical cues from perch (Perca fluviatilis). Oecologia 111: 166–171.
Agostinho, A. A., H. F. Julio-Junior, L. C. Gomes & L. M. Bini, 1997. Composição, abundância e distribuição espaço-temporal da ictiofauna. In Vazzoler, A. E. A. M., A. A. Agostinho & N. S. Hahn (eds), A Planície de inundação do alto rio Paraná: aspectos físicos, biológicos e socioeconômico. EDUEM, Maringá PR: 177–205.
Appelberg, M., B. Soderback & T. Odelstronl, 1993. Predator detection and perception of predation risk in the crayfish Astacus astacus L. Nordic Journal of Freshwater Research 68: 55–62.
Bell, R., A. L. Rypstra & M. H. Persons, 2006. The effect of predator hunger on chemically-mediated antipredator responses and survival in the wolf spider Pardosa milvina. Ethology 112: 903–910.
Brown, G. E. & G. Magnavacca, 2003. Predator inspection behaviour in a characin fish: an interaction between chemical and visual information? Ethology 109: 739–750.
Carlsson, N. O. L. & D. L. Strayer, 2009. Intraspecific variation in the consumption of exotic prey—a mechanism that increases biotic resistance against invasive species? Freshwater Biology 54: 2315–2319.
Carlsson, N. O. L., O. Sarnelle & D. L. Strayer, 2009. Native predators and exotic prey—an acquired taste? Frontiers in Ecology and the Environment 7: 525–532.
Cunha, E. R., S. M. Thomaz, H. B. A. Evangelista, C. Carniato, C. F. Souza & R. Fugi, 2011. Small-sized fish assemblages do not differ between a native and a recently established non-indigenous macrophyte in a Neotropical ecosystem. Natureza & Conservação 9: 61–66.
Dupuch, A., P. Magnan, A. Bertolo, L. M. Dill & M. Proulx, 2009. Does predation risk influence habitat use by northern redbelly dace Phoxinus eos at different spatial scales? Journal of Fish Biology 74: 1371–1382.
Ferrari, M. C. O., F. Messier & D. P. Chivers, 2008. Degradation of chemical alarm cues in the natural conditions: risk assessment by larval woodfrogs. Chemoecology 17: 263–266.
Figueiredo, B. R. S., R. P. Mormul & E. Benedito, 2013. Non-additive effects of macrophyte cover and turbidity on predator-prey interactions involving an invertivorous fish and different prey types. Hydrobiologia 716: 21–28.
Figueiredo, B. R. S., R. P. Mormul & E. Benedito, 2014. Structural complexity and turbidity do not interact to influence predation rate and prey selectivity by a small visually feeding fish. Marine and Freshwater Research. doi:10.1071/MF14030.
Gonzalo, A., C. Cabido, P. López & J. Martín, 2012. Conspecific alarm cues, but not predator cues alone, determine antipredator behavior of larval southern marbled newts, Triturus pygmaeus. Acta Ethologica 15: 211–216.
Goodman, B. A., 2009. Nowhere to run: the role of habitat openness and refuge use in defining patterns of morphological and performance evolution in tropical lizards. Journal of Evolutionary Biology 22: 1535–1544.
Helfman, G. S., 1989. Threat-sensitive predator avoidance in damselfish-trumpetfish interactions. Behavioral Ecology and Sociobiology 24: 47–58.
Holmes, T. H. & M. I. McCormick, 2011. Response across a gradient: behavioural reactions of newly settled fish to predation cues. Animal Behaviour 81: 543–550.
Hossie, T. J. & D. L. Murray, 2010. You can’t run but you can hide: refuge use in frog tadpoles elicits density-dependent predation by dragonfly larvae. Oecologia 163: 395–404.
Kovalenko, K. E., A. A. Agostinho, E. D. Dibble & F. M. Pelicice, 2010a. Recognition of non-native peacock bass, Cichla kelberi by native prey: testing the naiveté hypothesis. Biological Invasions 12: 3071–3080.
Kovalenko, K. E., E. D. Dibble, A. A. Agostinho, G. Catanhede & R. Fugi, 2010b. Direct and indirect effects of an introduced piscivore, Cichla kelberi and their modification by aquatic plants. Hydrobiologia 638: 245–253.
Lautala, T. & H. Hirvonen, 2008. Antipredator behavior of naïve Arctic charr young in the presence of predator odours and conspecific alarm cues. Ecology of Freshwater Fish 17: 78–85.
Luz-Agostinho, K. D. G., A. A. Agostinho, L. C. Gomes, H. F. Júlio-Jr. & R. Fugi, 2009. Effects of flooding regime on the feeding activity and body condition of piscivorous fish in the Upper Paraná River floodplain. Brazilian Journal of Biology 69: 481–490.
Orrock, J. L., E. L. Preisser, J. H. Grabowski & G. C. Trussell, 2013. The cost of safety: refuges increase the impact of predation risk in aquatic systems. Ecology 94: 573–579.
Pappal, A. L., R. A. Rountree & D. G. MacDonald, 2012. Relationship between body size and habitat complexity preference in age −0 and −1 year winter flounder Pseudopleuronectes americanus. Journal of Fish Biology 81: 220–229.
Pelicice, F. M. & A. A. Agostinho, 2009. Fish fauna destruction after the introduction of a non-native predator (Cichla kelberi) in a neotropical reservoir. Biological Invasions 11: 1789–1801.
Savino, J. F. & R. A. Stein, 1989. Behavior of fish predators and their prey: habitat choice between open water and dense vegetation. Environmental Biology of Fishes 24: 287–293.
Sousa, W. T. Z., 2011. Hydrilla verticillata (Hydrocharitaceae), a recent invader threatening Brazil’s freshwater environments: a review of the extent of the problem. Hydrobiologia 669: 1–20.
Acknowledgments
We thank two anonymous reviewers for comments made on our first draft. BRS Figueiredo is grateful to the Coordination for the Improvement of Higher Level Personnel (CAPES) for a scholarship. RP Mormul and SM Thomaz thank the National Council for Scientific and Technological Development (CNPq) for providing a post-doctoral research fellowship and a research productivity grant, respectively. Finally, we thank JVB Fasoli for help in the field and ER Cunha for providing valuable discussions. This work was partially supported by CAPES, an organ of the Brazilian Government for the training of human resources. The experiment was carried out in accordance with the “Ethical Principles in Animal Research” adopted by the Brazilian College of Animal Experimentation (COBEA).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Sidinei M. Thomaz, Katya E. Kovalenko, John E. Havel & Lee B. Kats / Aquatic Invasive Species
Rights and permissions
About this article
Cite this article
Figueiredo, B.R.S., Mormul, R.P. & Thomaz, S.M. Swimming and hiding regardless of the habitat: prey fish do not choose between a native and a non-native macrophyte species as a refuge. Hydrobiologia 746, 285–290 (2015). https://doi.org/10.1007/s10750-014-2096-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-014-2096-x