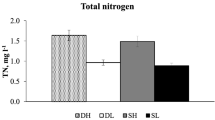
Abstract
Nutrient enrichment may alter population dynamics of species in different ways depending on their life strategies. The aim of this study was to test the effect of different nutrient concentrations on the population development of two bacterivorous freshwater nematodes, Bursilla monhystera and Plectus aquatilis. Microcosms with autoclaved natural sand from a pristine stream (Fuirosos, NE of Spain) were enriched with different levels of phosphate, nitrate and ammonia as inorganic nutrients and glucose as a biodegradable dissolved organic carbon source. Although leaching of carbon and nutrients from the detritus fraction in the sediment initially may have overruled differences between treatments, later samplings revealed bottom-up control, with Bursilla monhystera abundances positively correlated to bacterial abundances at high nutrient concentrations. Nevertheless, there were several indications that nematodes in turn affected microbial abundance, most likely through excretion of ammonia and through grazing. In contrast to B. monhystera, Plectus aquatilis at high nutrient concentrations showed a unimodal abundance curve, while not increasing in abundance at low nutrient concentrations. Glucose enrichment did not have any stimulatory effect on either microbial or nematode abundances, probably as a result of unfavourable C:N:P stoichiometry. P enrichment, by contrast, stimulated microbial and Bursilla abundances. Our results indicate that episodic nutrient enrichment may affect populations of bacterial-feeding nematodes in the short term. Their longer-term dynamics may, however, be more dependent on leaching of carbon and nutrients from the pools of sediment-bound detritus.





Similar content being viewed by others
References
Abebe, E., W. Decraemer & P. De Ley, 2008. Global diversity of nematodes (Nematoda) in freshwater. Developments in Hydrobiology 198: 67–78.
Abrams, B. I. & M. J. Mitchell, 1980. Role of nematode-bacterial interactions in heterotrophic systems with emphasis on sewage sludge decomposition. Oikos 35: 404–410.
Alkemade, R., A. Wielemaker & M. A. Hemminga, 1992a. Stimulation of Spartina anglica leaves by the bacterivorous marine nematode Diplolaimelloides bruciei. Journal of Experimental Marine Biology and Ecology 159: 267–278.
Alkemade, R., A. Wielemaker, S. A. de Jong & A. J. J. Sandee, 1992b. Experimental evidence for the role of bioturbation by the marine nematode Diplolaimella dievengatensis in stimulating the mineralization of Spartina anglica detritus. Marine Ecology Progress Series 90: 149–155.
Anderson, R. V. & D. C. Coleman, 1981. Population development and interactions between two species of bacteriophagic nematodes. Nematologica 27: 6–19.
Artigas, J., A. M. Romaní, A. Gaudes, I. Muñoz & S. Sabater, 2009. Organic matter availability structures microbial biomass and activity in a Mediterranean stream. Freshwater Biology 54: 2025–2036.
Benjamini, Y. & Y. Hochberg, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society 57: 289–300.
Bongers, T., 1999. The Maturity Index, the evolution of nematode life history traits, adaptive radiation and cp-scaling. Plant and Soil 212: 13–22.
Bonkowski, M., B. Griffiths & C. Scrimgeour, 2000. Substrate heterogeneity and microfauna in soil organic ‘hotspots’ as determinants of nitrogen capture and growth of ryegrass. Applied Soil Ecology 14: 37–53.
Bouwman, L. A., J. Bloem, P. H. J. F. van den Boogert, F. Bremer, G. H. J. Hoenderboem & P. C. de Ruiter, 1994. Short-term and long-term effects of bacterivorous nematodes and nematophagous fungi on carbon and nitrogen mineralization in microcosms. Biology and Fertility of Soils 17: 249–256.
Coleman, D. C., C. V. Cole, R. V. Anderson, M. Blaha, M. K. Campion, M. Clarholm, E. T. Elliott, H. W. Hunt, B. Shaefer & J. Sinclair, 1977. An analysis of rhizosphere-saprophage interactions in terrestrial ecosystems. In Lohm U. L. & T. Persson (eds), Soil Organisms as Components of Ecosystems. Ecological Bulletin Number 25, Swedish National Research Council. Stockholm: 299–309.
Cross, W. F., B. R. Johnson, J. B. Wallace & A. D. Rosemond, 2005. Contrasting response of stream detritivores to long-term nutrient enrichment. Limnology and Oceanography 50: 1730–1739.
Cross, W. F., J. B. Wallace & A. D. Rosemond, 2007. Nutrient enrichment reduces constraints on material flows in a detritus-based food web. Ecology 88: 2563–2575.
De Mesel, I., S. Derycke, J. Swings, M. Vincx & T. Moens, 2003. Influence of bacterivorous nematodes on the decomposition of cordgrass. Journal of Experimental Marine Biology and Ecology 296: 227–242.
De Mesel, I., S. Derycke, T. Moens, K. van der Gucht, M. Vincx & J. Swings, 2004. Top-down impact of bacterivorous nematodes on the bacterial community structure: a microcosm study. Environmental Microbiology 6: 733–744.
De Mesel, I., S. Derycke, J. Swings, M. Vincx & T. Moens, 2006. Role of nematodes in decomposition processes: does within-trophic group diversity matter? Marine Ecology Progress Series 321: 157–166.
Derycke, S., R. van Vynckt, J. Vanoverbeke, M. Vincx & T. Moens, 2007. Colonization patterns of Nematoda on decomposing algae in the estuarine environment: community assembly and genetic structure of the dominant species Pellioditis marina. Limnology and Oceanography 52: 992–1001.
Elser, J. J., & D. O. Hessen, 2005. Biosimplicity via stochiometry: the evolution of food-web structure and processes. In Belgrano, A., U. Scharler, J. Dunne & R. Ulanowicz (eds), Aquatic Food Webs: An Ecosystem Approach. Oxford University Press, UK.
Elser, J. J., K. Hayakawa & J. Urabe, 2001. Nutrient limitation reduces food quality for zooplankton: Daphnia response to seston phosphorus enrichment. Ecology 82: 898–903.
Feller, R. J. & R. M. Warwick, 1988. Energetics. In Higgins, R. P. & H. Thiel (eds), Introduction to the Study of Meiofauna. Smithsonian Institution. London, UK.
Ferris, H., S. S. Lau & R. C. Venette, 1995. Population energetics of bacterial-feeding nematodes: respiration and metabolic rates based on carbon dioxide production. Soil Biology and Biochemistry 27: 319–330.
Ferris, H., M. Eyre, R. C. Venette & S. S. Lau, 1996. Population energetics of bacterial-feeding nematodes: stage-specific development and fecundity rates. Soil Biology and Biochemistry 28: 271–280.
Ferris, H., R. C. Venette & S. S. Lau, 1997. Population energetics of bacterial-feeding nematodes: carbon and nitrogen budgets. Soil Biology and Biochemistry 29: 1183–1194.
Ferris, H., R. C. Venette, H. R. van der Meulen & S. S. Lau, 1998. Nitrogen mineralization by bacterial-feeding nematodes: verification and measurement. Plant and Soil 203: 159–171.
Findlay, S. & K. R. Tenore, 1982. Effect of a free-living marine nematode (Diplolaimella chitwoodi) on detrital carbon mineralization. Marine Ecology Progress Series 8: 161–166.
Freckman, D. W., 1988. Bacterivorous nematodes and organic-matter decomposition. Agriculture, Ecosystems and Environment 24: 195–217.
Gaudes, A., S. Sabater, E. Vilalta & I. Muñoz, 2006. The nematode community in cyanobacterial biofilms in the river Llobregat. Spain. Nematology. 8: 909–919.
Gaudes, A., J. Ocaña & I. Muñoz, 2012. Meiofaunal response to nutrient addition in a Mediterranean stream. Freshwater Biology. doi:10.1111/j.1365-2427.2012.02757.x
Greenwood, J. L., A. D. Rosemond, J. B. Wallace, W. F. Cross & H. S. Weyers, 2007. Nutrients stimulate leaf breakdown rates and detritivore biomass: bottom-up effects via heterotrophic pathways. Oecologia 151: 637–649.
Griffiths, B. S., 1994. Microbial-feeding nematodes and Protozoa in soil: their effects on microbial activity and nitrogen mineralization in decomposition hotspots and the rhizosphere. Plant and Soil 164: 25–33.
Gulis, V. & K. Suberkropp, 2003. Leaf litter decomposition and microbial activity in nutrient-enriched and unaltered reaches of a headwater stream. Freshwater Biology 48: 123–134.
Gulis, V., A. D. Rosemond, K. Suberkropp, H. S. Weyers & J. P. Benstead, 2004. Effects of nutrient enrichment on the decomposition of wood and associated microbial activity in streams. Freshwater Biology. 49: 1437–1447.
Heip, C., M. Vincx & G. Vranken, 1985. The ecology of marine nematodes. Oceanography and Marine Biology Annual Review 23: 399–489.
Hillebrand, H., M. Kahlert, A. L. Haglund, U. G. Berninger, S. Nagel & S. Wickham, 2002. Control of microbenthic communities by grazing and nutrient supply. Ecology 83: 2205–2219.
Hodda, M., 2006. Nematodes in lotic systems. In Abebe, E., W. Traunspurger & I. Andrássy (eds), Freshwater Nematodes: Ecology and Taxonomy. CABI Publishing. Oxfordshire, UK.
Hodda, M., A. Ocaña & W. Traunspurger, 2006. Nematodes from extreme freshwater habitats. In Abebe, E., W. Traunspurger & I. Andrássy (eds), Freshwater Nematodes: Ecology and Taxonomy. CABI Publishing. Oxfordshire, UK.
Höss, S., M. Bergtold, M. Haitzer, W. Traunspurger & C. E. W. Steinberg, 2001. Refractory dissolved organic matter can influence the reproduction of Caenorhabditis elegans (Nematoda). Freshwater Biology 46: 1–10.
Ingham, E. R., J. A. Trofymore, E. R. Ingham & D. C. Coleman, 1985. Interactions of bacteria, fungi, and their nematode grazers: effects on nutrient cycling and plant growth. Ecological Monographs 55: 119–140.
Johannes, R. E., 1965. Influence of marine protozoa on nutrient regeneration. Limnology and Oceanography 10: 434–442.
Kammenga, J. E., C. A. M. Van Gestel & J. Bakker, 1994. Patterns of sensitivity to cadmium and pentachlorophenol among nematode species from different taxonomic and ecological groups. Archives of Environmental Contamination and Toxicology 27: 88–94.
Lee, D. L. & H. J. Atkinson, 1977. Physiology of Nematodes. Columbia University Press, New York.
Lopez-Doval, J. C., M. Großschartner, S. Höss, C. Orendt, W. Traunspurger, G. Wolfram & I. Muñoz, 2010. Invertebrate communities in soft sediments along a pollution gradient in a Mediterranean river (Llobregat, NE Spain). Limnetica 29: 311–322.
Marchant, R. & W. L. Nicholas, 1974. An energy budget for the free-living nematode Pelodera (Rhabditidae). Oecologia 16: 237–252.
Martínez, J. G., G. A. P. dos Santos, S. Derycke & T. Moens, 2012. Effects of cadmium on the fitness of, and interactions between, two bacterivorous nematode species. Applied Soil Ecology 56: 10–18.
Moens, T. & M. Vincx, 1998. On the cultivation of free-living estuarine and marine nematodes. Helgol. Meeresunters 52: 115–139.
Moens, T., G. A. P. dos Santos, F. Thompson, J. Swings, V. Fonsêca-Genevois, M. Vincx & I. De Mesel, 2005. Do nematode mucus secretions affect bacterial growth? Aquatic Microbial Ecology 40: 77–83.
Postma-Blaauw, M. B., F. T. de Vries, R. G. de Goede, M. J. Bloem, J. H. Faber & L. Brussaard, 2005. Within-trophic group interactions of bacterivorous nematode species and their effects on the bacterial community and nitrogen mineralization. Oecologia 142: 428–439.
Riemann, F. & E. Helmke, 2002. Symbiotic relations of sediment-agglutinating nematodes and bacterial in detrital habitats: The Enzyme-Sharing Concept. Marine Ecology 23: 93–113.
Ristau, K. & W. Traunspurger, 2011. Relation between nematode communities and trophic state in southern Swedish lakes. Hydrobiologia 663: 121–133.
Sabater, S. & K. Tockner, 2010. Effects of hydrologic alterations in the ecological quality of river ecosystems. In Sabater, S. & D. Barceló (eds), Water Scarcity in Mediterranean Areas. Springer, Berlin
Sabater, S., J. Artigas, A. Gaudes, I. Muñoz, G. Urrea & A. M. Romaní, 2011. Longterm moderate nutrient inputs enhance autotrophy in a forested Mediterranean stream. Freshwater Biology. doi:10.1111/j.1365-2427.2010.02567.x.
Schiemer, F., 1982. Food dependence and energetics of freeliving nematodes. I. Respiration, growth and reproduction of Caenorhabditis briggsae (Nematoda) at different levels of food supply. Oecologia 54: 108–121.
Schiemer, F., 1983. Food dependence and energetics of free-living nematodes. III. Comparative aspects with special consideration of two bacterivorous species Caenorhabditis briggsae and Plectus palustris. Oikos 41: 32–43.
Schiemer, F., 1985. Bioenergetic niche differentiation of aquatic invertebrates. Verhandlungen der Internazionale Vereinigung für Theoretische und Angewandte Limnologie 22: 3014–3018.
Schroeder, F., W. Traunspurger, K. Pettersson & L. Peters, 2012. Temporal changes in periphytic meiofauna in lakes of different trophic states. Journal of Limnology 71: 216–227.
Sudhaus, W., 1980. Systematisch-phylogenetische und biologisch-ökologische Untersuchungen an Rhabditis- (Poikilolaimus-) Arten als Beitrag zur Rassenbildung und Parallelevolution bei Nematoden. Zool. Jb. syst. 107: 287–343.
Traunspurger, W., 2002. Nematoda. In Rundle, S. D., A. L. Robertson & J. M. Schmid-Araya (eds), Freshwater Meiofauna: Biology and Ecology. Backhuys Publishers. The Netherlands, Leiden.
Traunspurger, W., M. Bergtold & W. Goodkoep, 1997. The effect of nematodes on activity and abundance of bacteria in a profundal freshwater sediment. Oecologia 112: 118–122.
Venette, R. C. & H. Ferris, 1997. Thermal constraints to population growth of bacterial-feeding nematodes. Soil Biology and Biochemistry 29: 63–74.
Warwick, R. M. & R. Price, 1979. Ecological and metabolic studies on free-living nematodes from an estuarine mud-flat. Estuarine and Coastal Marine Science 9: 257–271.
Waters, T. F., 1977. Secondary production in inland waters. Advances in Ecological Research 10: 91–164.
Wieser, W., 1960. Benthic studies in Buzzards Bay II. The Meiofauna. Limnology and Oceanography 5: 121–137.
Woombs, M & J. Laybourn-Parry, 1985. Energy partitioning in three species of nematodes from polysaprobic environments. Oecologia 65: 289–295.
Yeates, G. W., 2003. Nematodes as soil indicators: functional and biodiversity aspects. Biology and Fertility of Soils 37: 199–210.
Acknowledgments
Financial support for the experiments reported here was obtained from Ghent University through BOF project 0110600002 from the Flemish Science Foundation FWO through project G.0192.09 and from University of Barcelona trough SCARCE-Consolider project from the Spanish Ministry. Claudia Höckelmann kindly provided the Lake Zürich samples from which the Plectus aquatilis culture was isolated. Esther Mas, Giovani P. dos Santos, Dirk Van Gansbeke and Tania Nara Bezerra also contributed to sample processing. We also thank the editor and two anonymous reviewers for their helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Stefano Amalfitano
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gaudes, A., Muñoz, I. & Moens, T. Bottom-up effects on freshwater bacterivorous nematode populations: a microcosm approach. Hydrobiologia 707, 159–172 (2013). https://doi.org/10.1007/s10750-012-1421-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1421-5