Abstract
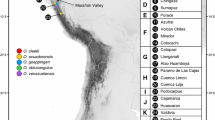
Darwinulid ostracods are putative ancient asexuals, and are thus assumed to be unable to purge deleterious mutations from their genomes. Some darwinulids species can be found both above (epigeic) and below ground (hypogeic). We hypothesize that surface populations carry more mutations than their below-ground counterparts, which are buffered from mutagens such as UV-B. Given the age of the investigated area, the Pilbara in Western Australia, we also expect geographic patterning of observed haplotypes. We have used DNA sequence data from the nuclear ITS and the mitochondrial COI region to investigate a (limited) data set on two Australian species, the endemic Vestalenula matildae and V. marmonieri from the Pilbara region. We do not find differences in genetic variability between specimens from subterranean habitats as compared to those from habitats above ground. There was also no congruence between hydrological basins and distribution patterns of the haplotypes identified. Although our data indicate that the two species may have split from each other ca. 70 myr ago, this has not resulted in any clear phylogeographic patterns among the analysed specimens across the regions of the Pilbara.





Similar content being viewed by others
References
Allwood, A. C., M. R. Walter, I. W. Burch & B. S. Kamber, 2007. 3.43 billion-year-old stromatolite reef from the Pilbara Craton of Western Australia: ecosystem-scale insights to early life on Earth. Precambrian Research 158: 198–227.
Altschul, S. F., W. Gish, W. Miller, E. W. Myers & D. J. Lipman, 1990. Basic local alignment search tool. Journal of Molecular Biology 215: 403–410.
Anonymous, 1984. Streamflow Records of Western Australia to 1982, 3 Vols. Public Works Department, Perth.
Artheau, M., 2007. Geographical review of the ostracod genus Vestalenula (Darwinulidae) and a new subterranean species from southern France. Invertebrate Systematics 21: 471–486.
Bell, G., 1982. The Masterpiece of Nature. Croom Helm, London.
Birky, C. W. Jr., 1996. Heterozygosity, heteromorphy, and phylogenetic trees in asexual eukaryotes. Genetics 144: 427–437.
Butlin, R. K., 2000. Virgin rotifers. Trends in Ecology & Evolution 15: 389–390.
Clement, M., D. Posada & K. A. Crandall, 2000. TCS: a computer program to estimate gene genealogies. Molecular Ecology 9: 1657–1660.
Eberhard, S. M., S. A. Halse, M. R. Williams, M. D. Scanlon, J. S. Cocking & H. J. Barron, 2007. Exploring the relationship between sampling efficiency and short range endemicity for stygofauna in the Pilbara region, Western Australia. Freshwater Biology 54: 885–901.
Finston, T. L., M. S. Johnson, W. F. Humphreys, S. M. Eberhard & S. A. Halse, 2007. Cryptic speciation in two widespread subterranean amphipod genera reflects historical drainage patterns in an ancient landscape. Molecular Ecology 16: 355–365.
Fisher, R. A., 1930. The Genetical Theory of Natural Selection. Clarendon Press, Oxford.
Folmer, O., M. Black, W. Hoeh, R. Lutz & R. Vrijenhoek, 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3: 294–299.
Frakes, L. A., B. McGowran & J. M. Bowler, 1981. Evolution of Australian environments. In George, A. S. (ed.), Flora of Australia Volume 1 – Introduction, 1st ed. Flora of Australia Series, CSIRO Publishing, Canberra: 1–14.
Guindon, S. & O. Gascuel, 2003. PhyML – a simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52: 696–704.
Hamilton, W. D., 1980. Sex versus non-sex versus parasite. Oikos 35: 282–290.
Hammer, Ø., D. A. T. Harper & P. D. Ryan, 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4: 9 pp.
Humphreys, W. F., 2008. Rising from Down Under: developments in subterranean biodiversity in Australia from a groundwater fauna perspective. Invertebrate Systematics 22: 85–101.
Karanovic, T., 2006. Subterranean copepods (Crustacea, Copepoda) from the Pilbara region in Western Australia. Records of the Western Australian Museum Supplement 70: 1–239.
Karanovic, I., 2007. Candoninae (Ostracoda) from the Pilbara region in Western Australia. Crustaceana Monographs 7: 1–432.
Knott, B. & S. A. Halse, 1999. Pilbarophreatoicus platyarthricus n.gen., n.sp. (Isopoda: Phreatoicidae: Amphisopodidae) from the Pilbara region of Western Australia. Records of the Australian Museum 51: 33–42.
Kondrashov, A. S., 1988. Deleterious mutations and the evolution of sexual reproduction. Nature 336: 435–440.
Kondrashov, A. S., 1993. Classification of hypotheses on the advantage of amphimixis. Journal of Heredity 84: 372–387.
Larkin, M. A., G. Blackshields, N. P. Brown, R. Chenna, P. A. McGettigan, H. McWilliam, F. Valentin, I. M. Wallace, A. Wilm, R. Lopez, J. D. Thompson, T. J. Gibson & D. G. Higgins, 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948.
Maddison, D. R. & W. P. Maddison, 2001. MacClade 4: Analysis of Phylogeny and Character Evolution. Version 4.02. Sinauer Associates, Sunderland, MA.
Mark Welch, D. & M. Meselson, 2000. Evidence for the evolution of bdelloid rotifers without sexual reproduction or genetic exchange. Science 288: 1211–1214.
Martens, K. (ed.), 1998. Sex and Parthenogenesis: Evolutionary Ecology of Reproductive Modes in Non-Marine Ostracods. Backhuys Publications, Leiden.
Martens, K. & G. Rossetti, 2002. On the Darwinulidae (Crustacea, Ostracoda) from Oceania, with the description of Vestalenula matildae n. sp. Invertebrate Systematics 16: 195–208.
Martens, K. & I. Schön, 2008. Opinion: ancient asexuals: darwinulids not exposed. Nature 453: 587.
Martens, K., G. Rossetti & D. J. Horne, 2003. How ancient are ancient asexuals? Proceedings of the Royal Society of London Series B 270: 723–729.
Martens, K., G. Rossetti, R. K. Butlin & I. Schön, 2005. Molecular and morphological phylogeny of the ancient asexual Darwinulidae (Crustacea, Ostracoda). Hydrobiologia 538: 153–165.
Martens, K., I. Schön, C. Meisch & D. J. Horne, 2008. Global diversity of ostracods (Ostracoda, Crustacea) in freshwater. Hydrobiologia 595: 185–193.
Martin, H. A., 2006. Cenozoic climatic change and the development of the arid vegetation in Australia. Journal of Arid Environments 66: 533–563.
Maynard Smith, J., 1980. Selection for recombination in a polygenic model. Genetic Research 35: 269–277.
Muller, H. J., 1932. Some genetic aspects of sex. American Naturalist 66: 118–138.
Muller, H. J., 1964. The relation of recombination to mutational advance. Mutational Research 1: 2–9.
Panchal, M., 2007. The automation of nested clade phylogeographic analysis. Bioinformatics 23: 509–510.
Pinto, R. L., C. E. F. Rocha & K. Martens, 2003. On two new species of the genus Vestalenula Rossetti & Martens, 1998 (Crustacea, Ostracoda, Darwinulidae) from semi-terrestrial habitats in Sao Paulo State (Brazil). Zoological Journal of the Linnean Society 139: 305–313.
Pinto, R. L., C. E. F. Rocha & K. Martens, 2004. On the genus Penthesilenula Rossetti & Martens, 1998 (Crustacea, Ostracoda, Darwinulidae) from (semi-) terrestrial habitats in São Paulo State (Brazil), with the description of a new species. Journal of Natural History 38: 2567–2589.
Posada, D., 2008. jModelTest: phylogenetic model averaging. Molecular Biology and Evolution 25: 1253–1256.
Posada, D., K. A. Crandall & A. R. Templeton, 2000. GeoDis: a program for the cladistic nested analysis of the geographical distribution of genetic haplotypes. Molecular Ecology 9: 487–488.
Rossetti, G. & K. Martens, 1998. Taxonomic revision of the Recent and Holocene representatives of the family Darwinulidae (Crustacea, Ostracoda), with a description of three new genera. Bulletin van het Koninklijk Belgisch Instituut voor Natuurwetenschappen Biologie 68: 55–110.
Roughgarden, J., 1991. The evolution of sex. American Naturalist 138: 934–953.
Schmidt, H. A., K. Strimmer, M. Vingron & A. von Haeseler, 2002. TREE-PUZZLE: maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics 18: 502–504.
Schön, I., 2001. Primers and PCR conditions for non-marine ostracods. BioTechniques 31: 1012–1016.
Schön, I., 2007. Did Pleistocene glaciations shape genetic patterns of European ostracods? A phylogeographic analysis of two species with asexual reproduction. Hydrobiologia 575: 33–50.
Schön, I. & K. Martens, 1998. Opinion: DNA-repair in an ancient asexual: a new solution to an old problem? Journal of Natural History 32: 943–948.
Schön, I. & K. Martens, 2003. No slave to sex. Proceedings of the Royal Society London Series B 270: 827–833.
Schön, I., R. K. Butlin, H. I. Griffiths & K. Martens, 1998. Slow molecular evolution in an ancient asexual ostracod. Proceedings of the Royal Society London Series B 265: 235–242.
Schön, I., K. Martens, K. Van Doninck & R. K. Butlin, 2003. Evolution in the slow lane: molecular rates of evolution in sexual and asexual ostracods (Crustacea: Ostracoda). Biological Journal of the Linnean Society 79: 93–100.
Schön, I., D. Lamatsch & K. Martens, 2008. Lessons to learn from ancient asexuals. In Egel, R. & D.-H. Lankenau (eds), Genomic Dynamics and Stability, Vol. 2. Meiosis and Recombination. Crossing Over and Disjunction. Springer Publishers, Berlin: 341–376.
Schön, I., G. Rossetti & K. Martens, 2009. Ancient asexual darwinulids: ancient scandals or scandalous gossip? In Schön, I., K. Martens & P. Van Dijk (eds), Lost Sex. The Evolutionary Biology of Parthenogenesis. Springer Academic Publishers, Dordrecht: 217–240.
Schurko, A. M., M. Neiman & J. M. Logsdon, 2009. Signs of sex; what we know and how we know it. Trends in Ecology & Evolution 24: 208–217.
Smith, R. J., T. Kamiya & D. J. Horne, 2006. Living males of the ‘ancient’ asexual Darwinulidae (Ostracoda, Crustacea). Proceedings of the Royal Society London Series B 273: 1569–1578.
Swofford, D. L., 1998. PAUP. Phylogenetic Analysis Using Parsimony (and Other Methods), Version 4.0. Sunderland Associates, Sunderland, MA.
Templeton, A. R., 1998. Nested clade analyses of phylogeographic data: testing hypotheses about gene flow and population history. Molecular Ecology 7: 381–397.
Van Doninck, K., I. Schön, L. De Bruyn & K. Martens, 2002. A general purpose genotype in an ancient asexual. Oecologia 132: 205–212.
Van Doninck, K., I. Schön, F. Maes, L. De Bruyn & K. Martens, 2003. Ecological strategies in the ancient asexual animal group Darwinulidae. Freshwater Biology 48: 1285–1294.
Van Valen, L. M., 1973. A new evolutionary law. Evolutionary Theory 1: 1–30.
White, T. J., T. Bruns, S. Lee & J. Taylor, 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In Innis, M. A., D. G. Gelfand, J. J. Sninsky & T. J. White (eds), PCR Protocols: A Guide to Methods and Applications. Academic Press, London: 315–322.
Acknowledgements
IS thanks the Edith Cowan University, Perth, Western Australia, for two fellowships as visiting fellow in 2006 and 2008/2009. KM and IS are grateful to the Department of Environment and Conservation and Bennelongia for financial support during their two scientific stays in Perth (2006 and 2008/2009). KM and IS also acknowledge the Belgian Belspo (project MO/36/015) for funding and financial contribution of the project 1.5.172.09 (krediet aan navorsers) of the FWO Vlaanderen (Fund for Scientific Research, Flanders).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Luigi Naselli-Flores
Rights and permissions
About this article
Cite this article
Schön, I., Martens, K. & Halse, S. Genetic diversity in Australian ancient asexual Vestalenula (Ostracoda, Darwinulidae): little variability down under. Hydrobiologia 641, 59–70 (2010). https://doi.org/10.1007/s10750-009-0057-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-009-0057-6