Abstract
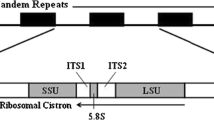
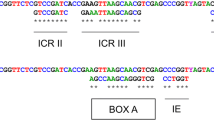
Nuclear ribosomal DNAs (rDNA) constitute a multi-gene family with tandemly arranged units linked by an intergenic spacer (IGS). Here we present the complete DNA sequence (7,731 bp) of a single repeat unit of an rDNA sequence from the moon jelly Aurelia sp.1 (Cnidaria: Scypozoa). The tandemly repeated rDNA units consisted of coding and non-coding regions, whose arrangement was 18S rDNA (1,814 bp, 46.2% of GC content)-internal transcribed spacer 1 (ITS1: 272 bp, 39.7%)-5.8S rDNA (158 bp; 50.7%)-ITS2 (278 bp, 51.4%)-28S rDNA (3,606 bp, 49.7%)-IGS (1,603 bp, 45.6%). GC composition in the single unit of rDNA was 47.8%. None of the 5S rDNA was found in the repeat units. Putative structures of a termination transcription signal (poly(T) tract) and promoter-like bi-repeats within the non-coding region were also identified. A block of minisatellites with five repeats was detected within the IGS. Comparative analyses of parsimony and dot plots showed that the IGS was highly informative. The sequence revealed here was the first completion of rDNA from the phylum Cnidaria, using as a model of rDNA for making molecular comparisons of jellyfish members.




Similar content being viewed by others
References
Arai, M. N., 1997. Functional biology of Scyphozoa. Chapman & Hall, New York, NY: 300.
Chen, C. A., D. J. Miller, N. V. Wei, C.-F. Dai & H.-P. Yang, 2000. The ETS/IGS region in a lower animal, the seawhip, Junceella fragilis (Cnidaria: Anthozoa: Octocorallia): compactness, low variation and apparent conservation of a pre-rRNA processing signal with fungi. Zoological Studies 39: 138–143.
Collins, A. G., P. Schuchert, A. C. Marques, T. Jankowski, M. Medina & B. Schierwater, 2006. Medusozoan phylogeny and character evolution clarified by new large and small subunit rDNA data and an assessment of the utility of phylogenetic mixture models. Systematic Biology 55: 97–115.
Dawson, M. N., 2003. Macro-morphological variation among cryptic species of the moon jellyfish, Aurelia (Cnidaria: Scyphozoa). Marine Biology 143: 369–379.
Dawson, M. N. & D. K. Jacobs, 2001. Molecular evidence for cryptic species of Aurelia aurita (Cnidaria, Scyphozoa). Biological Bulletin 200: 92–96.
Dawson, M. N. & L. E. Martin, 2001. Geographic variation and ecological adaptation in Aurelia (Scyphozoa: Semaeostomeae): some implications from molecular phylogenetics. Hydrobiologia 451: 259–273.
Dawson, M. N., A. Sen Gupta & M. H. England, 2005. Coupled biophysical global ocean model and molecular genetic analyses identify multiple introductions of cryptogenic species. Proceedings of the National Academy of Sciences of the United States of America 102: 11968–11973.
Drouin, G., J. D. Hofman & W. F. Doolittle, 1987. Unusual ribosomal RNA gene organization in copepods of the genus Calanus. Journal of Molecular Biology 196: 943–946.
Hassouna, N., B. Michot & J.-P. Bachellerie, 1984. The complete nucleotide sequence of mouse 28S rRNA gene. Implications for the process of size increase of the large subunit rRNA in higher eukaryotes. Nucleic Acids Research 12: 3563–3583.
Hillis, D. M. & M. T. Dixon, 1991. Ribosomal DNA: molecular evolution and phylogenetic inference. Quarterly Review of Biology 66: 411–453.
Jeong, S. W., W. H. Lang & R. H. Reeder, 1995. The release element of the yeast polymerase I transcription terminator can function independently of Reb1p. Molecular and Cellular Biology 15: 5929–5936.
Ki, J.-S. & M.-S. Han, 2007. Informative characteristics of 12 divergent domains in complete large subunit rDNA sequences from the harmful dinoflagellate genus, Alexandrium (Dinophyceae). Journal of Eukaryotic Microbiology 54: 210–219.
Ki, J.-S., D.-S. Hwang, K. Shin, W. D. Yoon, D. Lim, Y. S. Kang, Y. Lee & J. -S. Lee, 2008. Recent moon jelly (Aurelia sp.1) blooms in Korean coastal waters suggest global expansion: examples inferred from mitochondrial COI and nuclear ITS–5.8S rDNA sequences. ICES Journal of Marine Science 65: 443–452.
Kimura, M., 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution 16: 111–120.
Kramp, P. L., 1961. Synopsis of the medusae of the world. Journal of the Marine Biological Association of the United Kingdom 40: 337–342.
Kumar, S., K. Tamura, I. B. Jakobsen & M. Nei, 2001. MEGA3: Molecular Evolutionary Genetics Analysis software. Bioinformatics 17: 1244–1245.
Lang, W. H., B. E. Morrow, Q. Ju, J. R. Warner & R. H. Reeder, 1994. A model for transcription termination by RNA polymerase I. Cell 79: 527–534.
Lee, J.-S., 2000. The internally self-fertilizing hermaphroditic teleost Rivulus marmoratus (Cyprinodontiformes, Rivulidae) β-actin gene: amplification and sequence analysis with conserved primers. Marine Biotechnology 2: 161–166.
Marilley, M. & P. Pasero, 1996. Common DNA structural features exhibited by eukaryotic ribosomal gene promoters. Nucleic Acids Research 24: 2204–2211.
Mason, S., M. Wallisch & I. Grummt, 1997. RNA polymerase I transcription termination: similar mechanisms are employed by yeast and mammals. Journal of Molecular Biology 268: 229–234.
Mathews, D. H., J. Sabina, M. Zuker & D. H. Turner, 1999. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. Journal of Molecular Biology 288: 911–940.
Mayer, A. G., 1910. Medusae of the World, Vol. 3. The Scyphomedusae. Carnegie Institution of Washington, Washington, DC: 603–630.
Schlötterer, C., 1998. Ribosomal DNA probes and primers. In Karp, A., P. G. Isaac & D. S. Ingram (eds), Molecular Tools for Screening Biodiversity. Chapman & Hall, London: 267–276.
Schroth, W., G. Jarms, B. Streit & B. Schierwater, 2002. Speciation and phylogeography in the cosmopolitan marine moon jelly, Aurelia sp. BMC Evolutionary Biology 2: 1–10.
Zuker, M., 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Research 31: 3406–3415.
Acknowledgments
We would like to thank Drs. Sheikh Raisuddin and Hans-Uwe Dahms for English editing on the manuscript. We are very grateful to Dr. Timothy J. Page and two anonymous reviewers for reading and critical comments. This work was supported by grants of KOSEF NRL (2006) and ETEP (2006) funded to Jae-Seong Lee, and by a grant of KOSEF (2007) funded to Heum Gi Park. Thus work was also supported by a grant from Korea Polar Research Institute (PE08050) funded to Il-Chan Kim.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: K. A. Pitt & J. E. Purcell
Jellyfish Blooms: Causes, Consequences, and Recent Advances
Rights and permissions
About this article
Cite this article
Ki, JS., Kim, IC. & Lee, JS. Comparative analysis of nuclear ribosomal DNA from the moon jelly Aurelia sp.1 (Cnidaria: Scyphozoa) with characterizations of the 18S, 28S genes, and the intergenic spacer (IGS). Hydrobiologia 616, 229–239 (2009). https://doi.org/10.1007/s10750-008-9596-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-008-9596-5