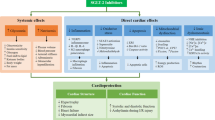
Abstract
This paper highlights the cardioprotective potential of sodium-glucose cotransporter 2 inhibitors (SLGT2i), as well as several most discussed mechanisms responsible for their cardioprotection. Cardiovascular diseases are considered a primary cause of death in nearly 80% of type 2 diabetes mellitus (T2DM) patients, with a 2–4-fold greater incidence of heart failure (HF) among diabetics. As novel hypoglycemics, SGLT2i showed exceptional cardiovascular benefits, reflected through robust reductions of cardiovascular mortality and hospitalization for HF in T2DM patients. Recently, those effects have been reported even in patients with HF and reduced ejection fraction irrespectively of diabetic status, suggesting that cardioprotective effects of SGLT2i are driven independently of their hypoglycemic actions. SGLT2i exerted hemodynamic and metabolic effects, partially driven by natriuresis and osmotic diuresis. However, those systemic effects are modest, and therefore cannot be completely related to the cardiac benefits of these agents in T2DM patients. Hence, increased circulating ketone levels during SGLT2i administration have brought out another hypothesis of a cardiac metabolic switch. Moreover, SGLT2i influence ion homeostasis and exert anti-inflammatory and antifibrotic effects. Their enviable influence on oxidative stress markers, as well as anti- and pro-apoptotic factors, have also been reported. However, since the main mechanistical contributor of their cardioprotection has not been elucidated yet, a joint action of systemic and molecular mechanisms has been suggested. In the light of ongoing trials evaluating the effects of SGLT2i in patients with HF and preserved ejection fraction, a new chapter of beneficial SGLT2i mechanisms is expected, which might resolve their main underlying action.
Similar content being viewed by others
References
Roth GA, Johnson C, Abajobir A et al (2017) Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol 70:1–25. https://doi.org/10.1016/j.jacc.2017.04.052
McAloon CJ, Osman F, Glennon P, Lim PB, Hayat SA (2016) Global epidemiology and incidence of cardiovascular disease. In: Papageorgiou N (ed) Cardiovascular disease—genetic susceptibility, enviromental factors and their interaction. Elsevier, Academic Press, The Netherlands, pp 57–96
Katsiki N, Banach M, Mikhailidis DP (2019) Is type 2 diabetes mellitus a coronary heart disease equivalent or not? do not just enjoy the debate and forget the patient! Arch Med Sci 15:1357–1364. https://doi.org/10.5114/aoms.2019.89449
Henning RJ (2018) Type-2 diabetes mellitus and cardiovascular disease. Future Cardiol 14:491–509. https://doi.org/10.2217/fca-2018-0045
Dunlay SM, Givertz MM, Aguilar D et al (2019) Type 2 diabetes mellitus and heart failure: a scientific statement from the American Heart Association and the Heart Failure Society of America: this statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation 140:e294–e324. https://doi.org/10.1161/CIR.0000000000000691
Echouffo-Tcheugui JB, Xu H, DeVore AD et al (2016) Temporal trends and factors associated with diabetes mellitus among patients hospitalized with heart failure: findings from get with the guidelines-heart failure registry. Am Heart J 182:9–20. https://doi.org/10.1016/j.ahj.2016.07.025
Ponikowski P, Voors AA, Anker SD et al (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37:2129–2200. https://doi.org/10.1093/eurheartj/ehw128
Rydén L, Grant PJ, Anker SD et al (2013) ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J 34:3035–3087. https://doi.org/10.1093/eurheartj/eht108
Bergmark BA, Bhatt DL, McGuire DK et al (2019) Metformin use and clinical outcomes among patients with diabetes mellitus with or without heart failure or kidney dysfunction: observations from the SAVOR-TIMI 53 trial. Circulation 140:1004–1014. https://doi.org/10.1161/CIRCULATIONAHA.119.040144
Douros A, Yin H, Yu OHY, Filion KB, Azoulay L, Suissa S (2017) Pharmacologic differences of sulfonylureas and the risk of adverse cardiovascular and hypoglycemic events. Diabetes Care 40:1506–1513. https://doi.org/10.2337/dc17-0595
Tentolouris A, Vlachakis P, Tzeravini E, Eleftheriadou I, Tentolouris N (2019) SGLT2 inhibitors: a review of their antidiabetic and cardioprotective effects. Int J Environ Res Public Health 16:2965. https://doi.org/10.3390/ijerph16162965
Ferrannini E, Solini A (2012) SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol 8:495–502. https://doi.org/10.1038/nrendo.2011.243
Khunti K, Davies M, Majeed A, Thorsted BL, Wolden ML, Paul SK (2015) Hypoglycemia and risk of cardiovascular disease and all-cause mortality in insulin-treated people with type 1 and type 2 diabetes: a cohort study. Diabetes Care 38:316–322. https://doi.org/10.2337/dc14-0920
Inzucchi SE, Docherty K, Kober L et al (2020) 271-OR: ADA Presidents’ select abstract: effect of dapagliflozin on the incidence of diabetes: a prespecified exploratory analysis from DAPA-HF. Diabetes 69:271-OR. https://doi.org/10.2337/db20-271-OR
Diamond GA, Bax L, Kaul S et al (2007) Uncertain effects of rosiglitazone on the risk for myocardial infarction and cardiovascular death. Ann Intern Med 147:578–581. https://doi.org/10.7326/0003-4819-147-8-200710160-00182
Cowie MR, Fisher M (2020) SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardio. https://doi.org/10.1038/s41569-020-0406-8
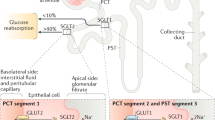
Gerich JE (2010) Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med 27:136–142. https://doi.org/10.1111/j.1464-5491.2009.02894.x
Vallon V, Thomson SC (2017) Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia 60:215–225. https://doi.org/10.1007/s00125-016-4157-3
Mather A, Pollock C (2011) Glucose handling by the kidney. Kidney Int Suppl 120:S1–S6. https://doi.org/10.1038/ki.2010.509
Kanai Y, Lee WS, You G, Brown D, Hediger MA (1994) The human kidney low affinity Na+/glucose cotransporter SGLT2. delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest 93:397–404. https://doi.org/10.1172/JCI116972
Mosley JF 2nd, Smith L, Everton E, Fellner C (2015) Sodium-glucose linked transporter 2 (SGLT2) inhibitors in the management of type-2 diabetes: a drug class overview. P T 40:451–462
DeFronzo RA, Norton L, Abdul-Ghani M et al (2017) Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol 13:11–26. https://doi.org/10.1038/nrneph.2016.170
Ehrenkranz JR, Lewis NG, Kahn CR, Roth J (2005) Phlorizin: a review. Diabetes Metab Res Rev 21:31–38. https://doi.org/10.1002/dmrr.532
Blaschek W (2017) Natural products as lead compounds for sodium glucose cotransporter (SGLT) inhibitors. Planta Med 83:985–993. https://doi.org/10.1055/s-0043-106050
Rieg T, Vallon V (2018) Development of SGLT1 and SGLT2 inhibitors. Diabetologia 61:2079–2086. https://doi.org/10.1007/s00125-018-4654-7
Cinti F, Moffa S, Impronta F, Cefalo CMA, Sun VA, Sorice GP, Mezza T, Giaccari A (2017) Spotlight on ertugliflozin and its potential in the treatment of type 2 diabetes: evidence to date. Drug Des Devel Ther 11:2905–2919. https://doi.org/10.2147/DDDT.S114932
Zinman B, Wanner C, Lachin JM et al (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373:2117–2128. https://doi.org/10.1056/NEJMoa1504720
Neal B, Perkovic V, Mahaffey KW et al (2017) Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377:644–657. https://doi.org/10.1056/NEJMoa1611925
Wiviott SD, Raz I, Bonaca MP et al (2019) Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 380:347–357. https://doi.org/10.1056/NEJMoa1812389
American Diabetes Association (2020) Standards of medical care in diabetes-2020 abridged for primary care providers. Clin Diabetes 38:10–38. https://doi.org/10.2337/cd20-as01
Zelniker TA, Wiviott SD, Raz I et al (2019) SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393:31–39. https://doi.org/10.1016/S0140-6736(18)32590-X
Kosiborod M, Cavender MA, Fu AZ et al (2017) Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium-glucose cotransporter-2 inhibitors). Circulation 136:249–259. https://doi.org/10.1161/CIRCULATIONAHA.117.029190
Kosiborod M, Lam CSP, Kohsaka S et al (2018) Cardiovascular events associated with SGLT-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL 2 study. J Am Coll Cardiol 71:2628–2639. https://doi.org/10.1016/j.jacc.2018.03.009
Patorno E, Pawar A, Franklin JM et al (2019) Empagliflozin and the risk of heart failure hospitalization in routine clinical care. Circulation 139:2822–2830. https://doi.org/10.1161/CIRCULATIONAHA.118.039177
Fitchett D, Zinman B, Wanner C et al (2016) Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J 37:1526–1534. https://doi.org/10.1093/eurheartj/ehv728
Fitchett D, Inzucchi SE, Cannon CP et al (2019) Empagliflozin reduced mortality and hospitalization for heart failure across the spectrum of cardiovascular risk in the EMPA-REG OUTCOME trial. Circulation 139:1384–1395. https://doi.org/10.1161/CIRCULATIONAHA.118.037778
Figtree GA, Rådholm K, Barrett TD et al (2019) Effects of canagliflozin on heart failure outcomes associated with preserved and reduced ejection fraction in type 2 diabetes mellitus. Circulation 139:2591–2593. https://doi.org/10.1161/CIRCULATIONAHA.119.040057
Kato ET, Silverman MG, Mosenzon O et al (2019) Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation 139:2528–2536. https://doi.org/10.1161/CIRCULATIONAHA.119.040130
McMurray JJV, Solomon SD, Inzucchi SE et al (2019) Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 381:1995–2008. https://doi.org/10.1056/NEJMoa1911303
U.S. Food and Drug Administration (2020) FDA approves new treatment for a type of heart failure. https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-type-heart-failure. Accessed 05 May 2020
Packer M, Anker SD, Butler J et al (2020) Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. https://doi.org/10.1056/NEJMoa2022190
Ilieșiu AM, Hodorogea AS (2018) Treatment of heart failure with preserved ejection fraction. Adv Exp Med Biol 1067:67–87. https://doi.org/10.1007/5584_2018_149
Packer M (2019) Lessons learned from the DAPA-HF trial concerning the mechanisms of benefit of SGLT2 inhibitors on heart failure events in the context of other large-scale trials nearing completion. Cardiovasc Diabetol 18:129. https://doi.org/10.1186/s12933-019-0938-6
Cannon CP (2007) Cardiovascular disease and modifiable cardiometabolic risk factors 8(3):11–28. https://doi.org/10.1016/s1098-3597(07)80025-1
Shaikh A (2017) A practical approach to hypertension management in diabetes. Diabetes Ther 8:981–989. https://doi.org/10.1007/s13300-017-0310-3
Tikkanen I, Chilton R, Johansen OE et al (2016) Potential role of sodium glucose cotransporter 2 inhibitors in the treatment of hypertension. Curr Opin Nephrol Hypertens 25:81–86. https://doi.org/10.1097/MNH.0000000000000199
Chen HY, Huang JY, Siao WZ, Jong GP (2020) The association between SGLT2 inhibitors and new-onset arrhythmias: a nationwide population-based longitudinal cohort study. Cardiovasc Diabetol 19:73. https://doi.org/10.1186/s12933-020-01048-x
Maliha G, Townsend RR (2015) SGLT2 inhibitors: their potential reduction in blood pressure. J Am Soc Hypertens 9:48–53. https://doi.org/10.1016/j.jash.2014.11.001
Zaccardi F, Webb DR, Htike ZZ, Youssef D, Khunti K, Davies MJ (2016) Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: systematic review and network meta-analysis. Diabetes Obes Metab 18:783–794. https://doi.org/10.1111/dom.12670
Bolinder J, Ljunggren Ö, Kullberg J, Johansson L, Wilding J, Langkilde AM, Sugg J, Parikh S (2012) Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 97:1020–1031. https://doi.org/10.1210/jc.2011-2260
Schork A, Saynisch J, Vosseler A et al (2019) Effect of SGLT2 inhibitors on body composition, fluid status and renin-angiotensin-aldosterone system in type 2 diabetes: a prospective study using bioimpedance spectroscopy. Cardiovasc Diabetol 18:46. https://doi.org/10.1186/s12933-019-0852-y
Cefalu WT, Stenlöf K, Leiter LA et al (2015) Effects of canagliflozin on body weight and relationship to HbA1c and blood pressure changes in patients with type 2 diabetes. Diabetologia 58:1183–1187. https://doi.org/10.1007/s00125-015-3547-2
Chilton R, Tikkanen I, Cannon CP, Crowe S, Woerle HJ, Broedl UC, Johansen OE (2015) Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab 17:1180–1193. https://doi.org/10.1111/dom.12572
O’Donnell M, Mente A, Yusuf S (2015) Sodium intake and cardiovascular health. Circ Res 116:1046–1057. https://doi.org/10.1161/CIRCRESAHA.116.303771
Schneider MP, Raff U, Kopp C et al (2017) Skin sodium concentration correlates with left ventricular hypertrophy in CKD. J Am Soc Nephrol 28:1867–1876. https://doi.org/10.1681/ASN.2016060662
Karg MV, Bosch A, Kannenkeril D et al (2018) SGLT-2-inhibition with dapagliflozin reduces tissue sodium content: a randomised controlled trial. Cardiovasc Diabetol 17:5. https://doi.org/10.1186/s12933-017-0654-z
Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI (2017) Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation 136:1643–1658. https://doi.org/10.1161/CIRCULATIONAHA.117.030012
Lopaschuk GD, Verma S (2020) Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl Sci 5:632–644. https://doi.org/10.1016/j.jacbts.2020.02.004
Pellicori P, Kaur K, Clark AL (2015) Fluid management in patients with chronic heart failure. Card Fail Rev 1:90–95. https://doi.org/10.15420/cfr.2015.1.2.90
Brown AJM, Gandy S, McCrimmon R, Houston JG, Struthers AD, Lang CC (2020) A randomized controlled trial of dapagliflozin on left ventricular hypertrophy in people with type two diabetes: the DAPA-LVH trial. Eur Heart J ehaa419. https://doi.org/10.1093/eurheartj/ehaa419
Verma S, Mazer CD, Yan AT et al (2019) Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA-HEART CardioLink-6 randomized clinical trial. Circulation 140:1693–1702. https://doi.org/10.1161/CIRCULATIONAHA.119.042375
Sattar N, McLaren J, Kristensen SL, Preiss D, McMurray JJ (2016) SGLT2 Inhibition and cardiovascular events: why did EMPA-REG Outcomes surprise and what were the likely mechanisms? Diabetologia 59:1333–1339. https://doi.org/10.1007/s00125-016-3956-x
Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J (2013) Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab 15:853–862. https://doi.org/10.1111/dom.12127
Mazer CD, Hare GMT, Connelly PW et al (2020) Effect of empagliflozin on Erythropoietin levels, iron stores, and red blood cell morphology in patients with type 2 diabetes mellitus and coronary artery disease. Circulation 141:704–707. https://doi.org/10.1161/CIRCULATIONAHA.119.044235
Takashima H, Yoshida Y, Nagura C, Furukawa T, Tei R, Maruyama T, Maruyama N, Abe M (2018) Renoprotective effects of canagliflozin, a sodium glucose cotransporter 2 inhibitor, in type 2 diabetes patients with chronic kidney disease: a randomized open-label prospective trial. Diab Vasc Dis Res 15:469–472. https://doi.org/10.1177/1479164118782872
Maruyama T, Takashima H, Oguma H et al (2019) Canagliflozin improves erythropoiesis in diabetes patients with anemia of chronic kidney disease. Diabetes Technol Ther 21:713–720. https://doi.org/10.1089/dia.2019.0212
Inzucchi SE, Zinman B, Fitchett D et al (2018) How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care 41:356–363. https://doi.org/10.2337/dc17-1096
Secrest MH, Udell JA, Filion KB (2017) The cardiovascular safety trials of DPP-4 inhibitors, GLP-1 agonists, and SGLT2 inhibitors. Trends Cardiovasc Med 27:194–202. https://doi.org/10.1016/j.tcm.2017.01.009
Scheen AJ, Paquot N (2014) Metabolic effects of SGLT-2 inhibitors beyond increased glucosuria: a review of the clinical evidence. Diabetes Metab 40(6 Suppl 1):S4–S11. https://doi.org/10.1016/S1262-3636(14)72689-8
Merovci A, Solis-Herrera C, Daniele G et al (2014) Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 124(2):509–514. https://doi.org/10.1172/JCI70704
Ferrannini E, Muscelli E, Frascerra S et al (2014) Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 124(2):499–508. https://doi.org/10.1172/JCI72227
Saponaro C, Pattou F, Bonner C et al (2018) SGLT2 inhibition and glucagon secretion in humans. Diabetes Metab 44(5):383–385. https://doi.org/10.1016/j.diabet.2018.06.005
Pereira MJ, Eriksson JW (2019) Emerging role of SGLT-2 inhibitors for the treatment of obesity. Drugs 79(3):219–230. https://doi.org/10.1007/s40265-019-1057-0
Lee PC, Ganguly S, Goh SY et al (2018) Weight loss associated with sodium-glucose cotransporter-2 inhibition: a review of evidence and underlying mechanisms. Obes Rev 19(12):1630–1641. https://doi.org/10.1111/obr.12755
Bays HE, Weinstein R, Law G, Canovatchel W (2014) Canagliflozin: effects in overweight and obese subjects without diabetes mellitus. Obesity (Silver Spring) 22(4):1042–1049. https://doi.org/10.1002/oby.20663
Scheen AJ (2019) Beneficial effects of SGLT2 inhibitors on fatty liver in type 2 diabetes: a common comorbidity associated with severe complications. Diabetes Metab 45(3):213–223. https://doi.org/10.1016/j.diabet.2019.01.008
Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW (2016) Non-alcoholic fatty liver disease and diabetes. Metabolism 65(8):1096–1108. https://doi.org/10.1016/j.metabol.2016.01.001
Targher G, Byrne CD, Tilg H et al (2020) NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut 69:1691–1705. https://doi.org/10.1136/gutjnl-2020-320622
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M (2016) Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64(1):73–84. https://doi.org/10.1002/hep.28431
Bhole V, Choi JW, Kim SW, de Vera M, Choi H (2010) Serum uric acid levels and the risk of type 2 diabetes: a prospective study. Am J Med 123(10):957–961. https://doi.org/10.1016/j.amjmed.2010.03.027
Zhao Y, Xu L, Tian D, Xia P, Zheng H, Wang L, Chen L (2018) Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: ameta-analysis of randomized controlled trials. Diabetes Obes Metab 20(2):458–462. https://doi.org/10.1111/dom.13101
Krishnan E (2009) Hyperuricemia and incident heart failure. Circ Heart Fail 2(6):556–562. https://doi.org/10.1161/CIRCHEARTFAILURE.108.797662
Gu J, Fan YQ, Zhang HL, Zhang JF, Wang CQ (2018) Serum uric acid is associated with incidence of heart failure with preserved ejection fraction and cardiovascular events in patients with arterial hypertension. J Clin Hypertens (Greenwich) 20(3):560–567. https://doi.org/10.1111/jch.13210
Saydah SH, Fradkin J, Cowie CC (2004) Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA 291(3):335–342. https://doi.org/10.1001/jama.291.3.335
Hayashi T, Fukui T, Nakanishi N et al (2017) Dapagliflozin decreases small dense low-density lipoprotein-cholesterol and increases high-density lipoprotein 2-cholesterol in patients with type 2 diabetes: comparison with sitagliptin [published correction appears in Cardiovasc Diabetol. et al 2017 Nov 13;16(1):149] Cardiovasc Diabetol 16 1 8 https://doi.org/10.1186/s12933-016-0491-5
Storgaard H, Gluud LL, Bennett C, Grøndahl MF, Christensen MB, Knop FK, Vilsbøll T (2016) Benefits and harms of sodium-glucose co-transporter 2 inhibitors in patients with type 2 diabetes: a systematic review and meta-analysis. PLoS One 11(11):e0166125. https://doi.org/10.1371/journal.pone.0166125
Filippas-Ntekouan S, Tsimihodimos V, Filippatos T, Dimitriou T, Elisaf M (2018) SGLT-2 inhibitors: pharmacokinetics characteristics and effects on lipids. Expert Opin Drug Metab Toxicol 14(11):1113–1121. https://doi.org/10.1080/17425255.2018.1541348
AMERICAN DIABETES ASSOCIATION (2003) Management of dyslipidemia in adults with diabetes. Diabetes Care 26(suppl 1):s83–s86. https://doi.org/10.2337/diacare.26.2007.S83
Aubert G, Martin OJ, Horton JL et al (2016) The failing heart relies on ketone bodies as a fuel. Circulation 133:698–705. https://doi.org/10.1161/CIRCULATIONAHA.115.017355
Staels B (2017) Cardiovascular protection by Sodium Glucose Cotransporter 2 Inhibitors: Potential Mechanisms. Am J Med 130:S30–S39. https://doi.org/10.1016/j.amjmed.2017.04.009
Nagoshi T, Yoshimura M, Rosano GM, Lopaschuk GD, Mochizuki S (2011) Optimization of cardiac metabolism in heart failure. Curr Pharm Des 17:3846–3853. https://doi.org/10.2174/138161211798357773
Maejima Y (2020) SGLT2 inhibitors play a salutary role in heart failure via modulation of the mitochondrial function. Front Cardiovasc Med 6:186. https://doi.org/10.3389/fcvm.2019.00186
Al Jobori H, Daniele G, Adams J, Cersosimo E, Triplitt C, DeFronzo RA, Abdul-Ghani M (2017) Determinants of the increase in ketone concentration during SGLT2 inhibition in NGT, IFG and T2DM patients. Diabetes Obes Metab 19:809–813. https://doi.org/10.1111/dom.12881
Santos-Gallego CG, Requena-Ibanez JA, San Antonio R et al (2019) Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol 73:1931–1944. https://doi.org/10.1016/j.jacc.2019.01.056
Oh CM, Cho S, Jang JY et al (2019) Cardioprotective potential of an SGLT2 inhibitor against doxorubicin-induced heart failure. Korean Circ J 49:1183–1195. https://doi.org/10.4070/kcj.2019.0180
Ferrannini E, Mark M, Mayoux E (2016) CV Protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care 39:1108–1114. https://doi.org/10.2337/dc16-0330
Wakabayashi S, Hisamitsu T, Nakamura TY et al (2013) Regulation of the cardiac Na+/H+ exchanger in health and disease. J Mol Cell Cardiol 61:68–76. https://doi.org/10.1016/j.yjmcc.2013.02.007
Fenton RA, Poulsen SB, de la Mora CS, Soleimani M, Dominguez Rieg JA, Rieg T (2017) Renal tubular NHE3 is required in the maintenance of water and sodium chloride homeostasis. Kidney Int 92:397–414. https://doi.org/10.1016/j.kint.2017.02.001
Packer M (2017) Activation and inhibition of sodium-hydrogen exchanger is a mechanism that links the pathophysiology and treatment of diabetes mellitus with that of heart failure. Circulation 136:1548–1559. https://doi.org/10.1161/CIRCULATIONAHA.117.030418
Pessoa TD, Campos LC, Carraro-Lacroix L, Girardi AC, Malnic G (2014) Functional role of glucose metabolism, osmotic stress, and sodium-glucose cotransporter isoform-mediated transport on Na+/H+ exchanger isoform 3 activity in the renal proximal tubule. J Am Soc Nephrol 25:2028–2039. https://doi.org/10.1681/ASN.2013060588
Di Franco A, Cantini G, Tani A et al (2017) Sodium-dependent glucose transporters (SGLT) in human ischemic heart: a new potential pharmacological target. Int J Cardiol 243:86–90. https://doi.org/10.1016/j.ijcard.2017.05.032
Baartscheer A, Schumacher CA, Wüst RC, Fiolet JW, Stienen GJ, Coronel R, Zuurbier CJ (2017) Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia 60:568–573. https://doi.org/10.1007/s00125-016-4134-x
Iborra-Egea O, Santiago-Vacas E, Yurista SR et al (2019) Unraveling the molecular mechanism of action of Empagliflozin in heart failure with reduced ejection fraction with or without diabetes. JACC Basic Transl Sci 4:831–840. https://doi.org/10.1016/j.jacbts.2019.07.010
Uthman L, Baartscheer A, Bleijlevens B et al (2018) Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia 61:722–726. https://doi.org/10.1007/s00125-017-4509-7
Hammoudi N, Jeong D, Singh R et al (2017) Empagliflozin improves left ventricular diastolic dysfunction in a genetic model of type 2 diabetes. Cardiovasc Drugs Ther 31:233–246. https://doi.org/10.1007/s10557-017-6734-1
Joubert M, Jagu B, Montaigne D et al (2017) The sodium-glucose cotransporter 2 inhibitor Dapagliflozin prevents cardiomyopathy in a diabetic lipodystrophic mouse model. Diabetes 66:1030–1040. https://doi.org/10.2337/db16-0733
Suarez J, Scott B, Dillmann WH (2008) Conditional increase in SERCA2a protein is able to reverse contractile dysfunction and abnormal calcium flux in established diabetic cardiomyopathy. Am J Physiol Regul Integr Comp Physiol 295:R1439–R1445. https://doi.org/10.1152/ajpregu.00736.2007
Trum M, Wagner S, Maier LS, Mustroph J (2020) CaMKII and GLUT1 in heart failure and the role of gliflozins. Biochim Biophys Acta Mol Basis Dis 1866:165729. https://doi.org/10.1016/j.bbadis.2020.165729
Schulman H, Anderson ME (2010) Ca/Calmodulin-dependent protein kinase II in heart failure. Drug Discov Today Dis Mech 7(2):e117–e122. https://doi.org/10.1016/j.ddmec.2010.07.005
Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM (2005) Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res 97:1314–1322. https://doi.org/10.1161/01.RES.0000194329.41863.89
Mustroph J, Wagemann O, Lücht CM, Trum M, Hammer KP, Martin C et al (2018) Empagliflozin reduces Ca/calmodulin-dependent kinase II activity in isolated ventricular cardiomyocytes. ESC Hear Fail 5:642–648. https://doi.org/10.1002/ehf2.12336
Suthahar N, Meijers WC, Silljé HHW, de Boer RA (2017) From inflammation to fibrosis-molecular and cellular mechanisms of myocardial tissue remodelling and perspectives on differential treatment opportunities. Curr Heart Fail Rep 14:235–250. https://doi.org/10.1007/s11897-017-0343-y
Bonnet F, Scheen AJ (2018) Effects of SGLT2 inhibitors on systemic and tissue low-grade inflammation: the potential contribution to diabetes complications and cardiovascular disease. Diabetes Metab 44:457–464. https://doi.org/10.1016/j.diabet.2018.09.005
Kelley N, Jeltema D, Duan Y, He Y (2019) The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci 20:3328. https://doi.org/10.3390/ijms20133328
Butts B, Gary RA, Dunbar SB, Butler J (2015) The importance of NLRP3 inflammasome in heart failure. J Card Fail 21:586–593. https://doi.org/10.1016/j.cardfail.2015.04.014
Kim SR, Lee SG, Kim SH et al (2020) SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun 11:2127. https://doi.org/10.1038/s41467-020-15983-6
Ye Y, Bajaj M, Yang HC, Perez-Polo JR, Birnbaum Y (2017) SGLT-2 inhibition with dapagliflozin reduces the activation of the Nlrp3/ASC inflammasome and attenuates the development of diabetic cardiomyopathy in mice with type 2 diabetes. Further augmentation of the effects with Saxagliptin, a DPP4 inhibitor. Cardiovasc Drugs Ther 31:119–132. https://doi.org/10.1007/s10557-017-6725-2
Byrne NJ, Matsumura N, Maayah ZH et al (2020) Empagliflozin blunts worsening cardiac dysfunction associated with reduced NLRP3 (nucleotide-binding domain-like receptor protein 3) inflammasome activation in heart failure. Circ Heart Fail 13:e006277. https://doi.org/10.1161/CIRCHEARTFAILURE.119.006277
Kusaka H, Koibuchi N, Hasegawa Y, Ogawa H, Kim-Mitsuyama S (2016) Empagliflozin lessened cardiac injury and reduced visceral adipocyte hypertrophy in prediabetic rats with metabolic syndrome. Cardiovasc Diabetol 15:157. https://doi.org/10.1186/s12933-016-0473-7
Lin B, Koibuchi N, Hasegawa Y et al (2014) Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc Diabetol 13:148. https://doi.org/10.1186/s12933-014-0148-1
Shi X, Verma S, Yun J et al (2017) Effect of empagliflozin on cardiac biomarkers in a zebrafish model of heart failure: clues to the EMPA-REG OUTCOME trial? Mol Cell Biochem 433:97–102. https://doi.org/10.1007/s11010-017-3018-9
Arow M, Waldman M, Yadin D et al (2020) Sodium-glucose cotransporter 2 inhibitor Dapagliflozin attenuates diabetic cardiomyopathy. Cardiovasc Diabetol 19:7. https://doi.org/10.1186/s12933-019-0980-4
Lee TM, Chang NC, Lin SZ (2017) Dapagliflozin, a selective SGLT2 Inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic Biol Med 104:298–310. https://doi.org/10.1016/j.freeradbiomed.2017.01.035
Lim CT, Kola B, Korbonits M (2010) AMPK as a mediator of hormonal signalling. J Mol Endocrinol 44:87–97. https://doi.org/10.1677/JME-09-0063
Coughlan KA, Valentine RJ, Ruderman NB, Saha AK (2014) AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes 7:241–253. https://doi.org/10.2147/DMSO.S43731
Fryer LG, Parbu-Patel A, Carling D (2002) The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem 277:25226–25232. https://doi.org/10.1074/jbc.M202489200
Hawley SA, Ford RJ, Smith BK et al (2016) The Na+/glucose cotransporter inhibitor canagliflozin activates AMPK by inhibiting mitochondrial function and increasing cellular AMP levels. Diabetes 65:2784–2794. https://doi.org/10.2337/db16-0058
Mancini SJ, Boyd D, Katwan OJ et al (2018) Canagliflozin inhibits interleukin-1β-stimulated cytokine and chemokine secretion in vascular endothelial cells by AMP-activated protein kinase-dependent and -independent mechanisms. Sci Rep 8:5276. https://doi.org/10.1038/s41598-018-23420-4
Zhou H, Wang S, Zhu P, Hu S, Chen Y, Ren J (2018) Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol 15:335–346. https://doi.org/10.1016/j.redox.2017.12.019
Lu Q, Liu J, Li X et al (2020) Empagliflozin attenuates ischemia and reperfusion injury through LKB1/AMPK signaling pathway. Mol Cell Endocrinol 501:110642. https://doi.org/10.1016/j.mce.2019.110642
Li C, Zhang J, Xue M et al (2019) SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol 18:15. https://doi.org/10.1186/s12933-019-0816-2
Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, Dhama K (2014) Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int 2014:761264. https://doi.org/10.1155/2014/761264
Asmat U, Abad K, Ismail K (2016) Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm J 24:547–553. https://doi.org/10.1016/j.jsps.2015.03.013
van der Pol A, van Gilst WH, Voors AA, van der Meer P (2019) Treating oxidative stress in heart failure: past, present and future. Eur J Heart Fail 21:425–435. https://doi.org/10.1002/ejhf.1320
Wilson AJ, Gill EK, Abudalo RA, Edgar KS, Watson CJ, Grieve DJ (2018) Reactive oxygen species signalling in the diabetic heart: emerging prospect for therapeutic targeting. Heart 104:293–299. https://doi.org/10.1136/heartjnl-2017-311448
Zhao QD, Viswanadhapalli S, Williams P et al (2015) NADPH oxidase 4 induces cardiac fibrosis and hypertrophy through activating Akt/mTOR and NFκB signaling pathways. Circulation 131:643–655. https://doi.org/10.1161/CIRCULATIONAHA.114.011079
Wu XY, Luo AY, Zhou YR, Ren JH (2014) N-acetylcysteine reduces oxidative stress, nuclear factor κB activity and cardiomyocyte apoptosis in heart failure. Mol Med Rep 10:615–624. https://doi.org/10.3892/mmr.2014.2292
Uthman L, Baartscheer A, Schumacher CA et al (2018) Direct cardiac actions of sodium glucose cotransporter 2 inhibitors target pathogenic mechanisms underlying heart failure in diabetic patients. Front Physiol 9:1575. https://doi.org/10.3389/fphys.2018.01575
Pickering RJ, Rosado CJ, Sharma A, Buksh S, Tate M, de Haan JB (2018) Recent novel approaches to limit oxidative stress and inflammation in diabetic complications. Clin Transl Immunology 7:e1016. https://doi.org/10.1002/cti2.1016
Terami N, Ogawa D, Tachibana H et al (2014) Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS One 9:e100777. https://doi.org/10.1371/journal.pone.0100777
Sayour AA, Korkmaz-Icöz S, Loganathan S et al (2019) Acute canagliflozin treatment protects against in vivo myocardial ischemia-reperfusion injury in non-diabetic male rats and enhances endothelium-dependent vasorelaxation. J Transl Med 17:127. https://doi.org/10.1186/s12967-019-1881-8
Steven S, Frenis K, Oelze M et al (2019) Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxid Med Cell Longev 2019:7092151. https://doi.org/10.1155/2019/7092151
Kay AM, Simpson CL, Stewart JA Jr et al (2016) The role of AGE/RAGE signaling in diabetes-mediated vascular calcification. J Diabetes Res 2016:6809703. https://doi.org/10.1155/2016/6809703
Oelze M, Kröller-Schön S, Welschof P et al (2014) The sodium-glucose co-transporter 2 inhibitor empagliflozin improves diabetes-induced vascular dysfunction in the streptozotocin diabetes rat model by interfering with oxidative stress and glucotoxicity. PLoS One 9:e112394. https://doi.org/10.1371/journal.pone.0112394
Tanajak P, Sa-Nguanmoo P, Sivasinprasasn S, Thummasorn S, Siri-Angkul N, Chattipakorn SC, Chattipakorn N (2018) Cardioprotection of dapagliflozin and vildagliptin in rats with cardiac ischemia-reperfusion injury. J Endocrinol 236:69–84. https://doi.org/10.1530/JOE-17-0457
Zhou Y, Wu W (2017) The sodium-glucose co-transporter 2 inhibitor, empagliflozin, protects against diabetic cardiomyopathy by inhibition of the endoplasmic reticulum stress pathway. Cell Physiol Biochem 41:2503–2512. https://doi.org/10.1159/000475942
Banerjee SK, McGaffin KR, Pastor-Soler NM, Ahmad F (2009) SGLT1 is a novel cardiac glucose transporter that is perturbed in disease states. Cardiovasc Res 84:111–118. https://doi.org/10.1093/cvr/cvp190
Filippatos TD, Liontos A, Papakitsou I, Elisaf MS (2019) SGLT2 inhibitors and cardioprotection: a matter of debate and multiple hypotheses. Postgrad Med 131:82–88. https://doi.org/10.1080/00325481.2019.1581971
Funding
This work was supported by Faculty of Medical Sciences, University of Kragujevac (Junior project No. 03/18 and No. 03/20).
Author information
Authors and Affiliations
Contributions
Maja Nikolic—collecting literature data, writing a manuscript and table design; Vladimir Zivkovic—text supervising and research the literature; Jovana Joksimovic Jovic—text supervising and editing; Jasmina Sretenovic—text supervising and editing; Goran Davidovic—design of the topic; Stefan Simovic—text supervising and editing; Danijela Djokovic—text supervising and final approval; Nemanja Muric—text supervising; Sergey Bolevich—text supervising and final approval; Vladimir Jakovljevic—text supervising and final approval.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nikolic, M., Zivkovic, V., Jovic, J.J. et al. SGLT2 inhibitors: a focus on cardiac benefits and potential mechanisms. Heart Fail Rev 27, 935–949 (2022). https://doi.org/10.1007/s10741-021-10079-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-021-10079-9