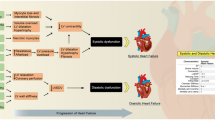
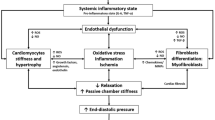
Abstract
Experimental models of cardiac disease play a key role in understanding the pathophysiology of the disease and developing new therapies. The features of the experimental models should reflect the clinical phenotype, which can have a wide spectrum of underlying mechanisms. We review characteristics of commonly used experimental models of cardiac physiology and pathophysiology in all translational steps including in vitro, small animal, and large animal models. Understanding their characteristics and relevance to clinical disease is the key for successful translation to effective therapies.
Similar content being viewed by others
References
Ziaeian B, Fonarow GC (2016) Epidemiology and aetiology of heart failure. Nat Rev Cardiol 13:368–378
Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA (2007) Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol 293:H1883–H1891
Fujiu K, Nagai R (2013) Contributions of cardiomyocyte-cardiac fibroblast-immune cell interactions in heart failure development. Basic Res Cardiol 108:357
Sequeira V, van der Velden J (2015) Historical perspective on heart function: the Frank-Starling Law. Biophys Rev 7:421–447
Brette F, Orchard C (2003) T-tubule function in mammalian cardiac myocytes. Circ Res 92:1182–1192
Chlopcikova S, Psotova J, Miketova P (2001) Neonatal rat cardiomyocytes--a model for the study of morphological, biochemical and electrophysiological characteristics of the heart. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 145:49–55
Muller-Werdan U, Klein D, Zander M, Werdan K, Hammer C (1994) Beating neonatal rat cardiomyocytes as a model to study the role of xenoreactive natural antibodies in xenotransplantation. Transplantation 58:1403–1409
Louch WE, Sheehan KA, Wolska BM (2011) Methods in cardiomyocyte isolation, culture, and gene transfer. J Mol Cell Cardiol 51:288–298
Djurovic S, Iversen N, Jeansson S, Hoover F, Christensen G (2004) Comparison of nonviral transfection and adeno-associated viral transduction on cardiomyocytes. Mol Biotechnol 28:21–32
Frank D, Kuhn C, Brors B et al (2008) Gene expression pattern in biomechanically stretched cardiomyocytes: evidence for a stretch-specific gene program. Hypertension 51:309–318
Harary I, Farley B (1963) In vitro studies on single beating rat heart cells. I. Growth and organization. Exp Cell Res 29:451–465
Mohamed BA, Barakat AZ, Zimmermann WH et al (2012) Targeted disruption of Hspa4 gene leads to cardiac hypertrophy and fibrosis. J Mol Cell Cardiol 53:459–468
Simpson P, McGrath A, Savion S (1982) Myocyte hypertrophy in neonatal rat heart cultures and its regulation by serum and by catecholamines. Circ Res 51:787–801
Huang Q, Huang J, Zeng Z, Luo J, Liu P, Chen S, Liu B, Pan X, Zang L, Zhou S (2015) Effects of ERK1/2/PPARalpha/SCAD signal pathways on cardiomyocyte hypertrophy induced by insulin-like growth factor 1 and phenylephrine. Life Sci 124:41–49
Nakaoka M, Iwai-Kanai E, Katamura M, Okawa Y, Mita Y, Matoba S (2015) An alpha-adrenergic agonist protects hearts by inducing Akt1-mediated autophagy. Biochem Biophys Res Commun 456:250–256
Zobel C, Kassiri Z, Nguyen TT, Meng Y, Backx PH (2002) Prevention of hypertrophy by overexpression of Kv4.2 in cultured neonatal cardiomyocytes. Circulation 106:2385–2391
Menaouar A, Florian M, Wang D, Danalache B, Jankowski M, Gutkowska J (2014) Anti-hypertrophic effects of oxytocin in rat ventricular myocytes. Int J Cardiol 175:38–49
Sadoshima J, Izumo S (1993) Molecular characterization of angiotensin II--induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res 73:413–423
Sakai S, Shimojo N, Kimura T, Tajiri K, Maruyama H, Homma S, Kuga K, Mizutani T, Aonuma K, Miyauchi T (2014) Involvement of peptidyl-prolyl isomerase Pin1 in the inhibitory effect of fluvastatin on endothelin-1-induced cardiomyocyte hypertrophy. Life Sci 102:98–104
Reid BG, Stratton MS, Bowers S et al (2016) Discovery of novel small molecule inhibitors of cardiac hypertrophy using high throughput, high content imaging. J Mol Cell Cardiol 97:106–113
Skwarek-Maruszewska A, Hotulainen P, Mattila PK, Lappalainen P (2009) Contractility-dependent actin dynamics in cardiomyocyte sarcomeres. J Cell Sci 122:2119–2126
Fan X, Hughes BG, Ali MA, Chan BY, Launier K, Schulz R (2016) Matrix metalloproteinase-2 in oncostatin M-induced sarcomere degeneration in cardiomyocytes. Am J Physiol Heart Circ Physiol 311:H183–H189
Komuro I, Kaida T, Shibazaki Y, Kurabayashi M, Katoh Y, Hoh E, Takaku F, Yazaki Y (1990) Stretching cardiac myocytes stimulates protooncogene expression. J Biol Chem 265:3595–3598
Komuro I, Katoh Y, Kaida T, Shibazaki Y, Kurabayashi M, Hoh E, Takaku F, Yazaki Y (1991) Mechanical loading stimulates cell hypertrophy and specific gene expression in cultured rat cardiac myocytes. Possible role of protein kinase C activation. J Biol Chem 266:1265–1268
Lin L, Tang C, Xu J et al (2014) Mechanical stress triggers cardiomyocyte autophagy through angiotensin II type 1 receptor-mediated p38MAP kinase independently of angiotensin II. PLoS One 9:e89629
Choudhary R, Baker KM, Pan J (2008) All-trans retinoic acid prevents angiotensin II- and mechanical stretch-induced reactive oxygen species generation and cardiomyocyte apoptosis. J Cell Physiol 215:172–181
Cheng TH, Chen JJ, Shih NL et al (2009) Mechanical stretch induces endothelial nitric oxide synthase gene expression in neonatal rat cardiomyocytes. Clin Exp Pharmacol Physiol 36:559–566
Wang BW, Wu GJ, Cheng WP, Shyu KG (2013) Mechanical stretch via transforming growth factor-beta1 activates microRNA-208a to regulate hypertrophy in cultured rat cardiac myocytes. J Formos Med Assoc 112:635–643
Liu W, Wang X, Mei Z, Gong J, Gao X, Zhao Y, Ma J, Xie F, Qian L (2015) Chronic stress promotes the progression of pressure overload-induced cardiac dysfunction through inducing more apoptosis and fibrosis. Physiol Res 64:325–334
Acosta D, Puckett M (1977) Ischemic myocardial injury in cultured heart cells: preliminary observations on morphology and beating activity. In Vitro 13:818–823
Wang XX, Wang XL, Tong MM, Gan L, Chen H, Wu SS, Chen JX, Li RL, Wu Y, Zhang HY, Zhu Y, Li YX, He JH, Wang M, Jiang W (2016) SIRT6 protects cardiomyocytes against ischemia/reperfusion injury by augmenting FoxO3alpha-dependent antioxidant defense mechanisms. Basic Res Cardiol 111:13
Peng K, Qiu Y, Li J, Zhang ZC, Ji FH (2017) Dexmedetomidine attenuates hypoxia/reoxygenation injury in primary neonatal rat cardiomyocytes. Exp Ther Med 14:689–695
Bagheri F, Khori V, Alizadeh AM, Khalighfard S, Khodayari S, Khodayari H (2016) Reactive oxygen species-mediated cardiac-reperfusion injury: mechanisms and therapies. Life Sci 165:43–55
Diaz RJ, Wilson GJ (2006) Studying ischemic preconditioning in isolated cardiomyocyte models. Cardiovasc Res 70:286–296
Graham RM, Frazier DP, Thompson JW et al (2004) A unique pathway of cardiac myocyte death caused by hypoxia-acidosis. J Exp Biol 207:3189–3200
Musters RJ, Post JA, Verkleij AJ (1991) The isolated neonatal rat-cardiomyocyte used in an in vitro model for ‘ischemia’. I. a morphological study. Biochim Biophys Acta 1091:270–277
Kim MY, Seo EJ, Lee DH, Kim EJ, Kim HS, Cho HY, Chung EY, Lee SH, Baik EJ, Moon CH, Jung YS (2010) Gadd45beta is a novel mediator of cardiomyocyte apoptosis induced by ischaemia/hypoxia. Cardiovasc Res 87:119–126
Tu S, Liu ZQ, Fu JJ, Zhu WF, Luo DY, Wan FS (2012) Inhibitory effect of p53 upregulated modulator of apoptosis targeting siRNA on hypoxia/reoxygenation-induced cardiomyocyte apoptosis in rats. Cardiology 122:93–100
Gilsbach R, Preissl S, Gruning BA et al (2014) Dynamic DNA methylation orchestrates cardiomyocyte development, maturation and disease. Nat Commun 5:5288
Dolinsky VW, Soltys CL, Rogan KJ et al (2015) Resveratrol prevents pathological but not physiological cardiac hypertrophy. J Mol Med (Berl) 93:413–425
von Lueder TG, Wang BH, Kompa AR et al (2015) Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ Heart Fail 8:71–78
Bell RM, Mocanu MM, Yellon DM (2011) Retrograde heart perfusion: the Langendorff technique of isolated heart perfusion. J Mol Cell Cardiol 50:940–950
Tan T, Marin-Garcia J, Damle S, Weiss HR (2010) Hypoxia-inducible factor-1 improves inotropic responses of cardiac myocytes in ageing heart without affecting mitochondrial activity. Exp Physiol 95:712–722
Mellor KM, Curl CL, Chandramouli C, Pedrazzini T, Wendt IR, Delbridge LM (2014) Ageing-related cardiomyocyte functional decline is sex and angiotensin II dependent. Age (Dordr) 36:9630
Wang YX, Korth M (1995) Effects of doxorubicin on excitation-contraction coupling in Guinea pig ventricular myocardium. Circ Res 76:645–653
Oh JG, Kim J, Jang SP et al (2013) Decoy peptides targeted to protein phosphatase 1 inhibit dephosphorylation of phospholamban in cardiomyocytes. J Mol Cell Cardiol 56:63–71
Zhang M, Prosser BL, Bamboye MA et al (2015) Contractile function during angiotensin-II activation: increased Nox2 activity modulates cardiac calcium handling via phospholamban phosphorylation. J Am Coll Cardiol 66:261–272
Toischer K, Zhu W, Hunlich M et al (2017) Cardiomyocyte proliferation prevents failure in pressure overload but not volume overload. J Clin Invest 127:4285–4296
Du CK, Morimoto S, Nishii K et al (2007) Knock-in mouse model of dilated cardiomyopathy caused by troponin mutation. Circ Res 101:185–194
Inoue T, Kobirumaki-Shimozawa F, Kagemoto T, Fujii T, Terui T, Kusakari Y, Hongo K, Morimoto S, Ohtsuki I, Hashimoto K, Fukuda N (2013) Depressed Frank-Starling mechanism in the left ventricular muscle of the knock-in mouse model of dilated cardiomyopathy with troponin T deletion mutation DeltaK210. J Mol Cell Cardiol 63:69–78
Hu LR, Ackermann MA, Hecker PA et al (2017) Deregulated ca(2+) cycling underlies the development of arrhythmia and heart disease due to mutant obscurin. Sci Adv 3:e1603081
Bhargava A, Lin X, Novak P et al (2013) Super-resolution scanning patch clamp reveals clustering of functional ion channels in adult ventricular myocyte. Circ Res 112:1112–1120
Gaitas A, Malhotra R, Li T, Herron T, Jalife J (2015) A device for rapid and quantitative measurement of cardiac myocyte contractility. Rev Sci Instrum 86:034302
Moshal KS, Tipparaju SM, Vacek TP, Kumar M, Singh M, Frank IE, Patibandla PK, Tyagi N, Rai J, Metreveli N, Rodriguez WE, Tseng MT, Tyagi SC (2008) Mitochondrial matrix metalloproteinase activation decreases myocyte contractility in hyperhomocysteinemia. Am J Physiol Heart Circ Physiol 295:H890–H897
Cagalinec M, Waczulikova I, Ulicna O, Chorvat D Jr (2013) Morphology and contractility of cardiac myocytes in early stages of streptozotocin-induced diabetes mellitus in rats. Physiol Res 62:489–501
Kerr JP, Robison P, Shi G et al (2015) Detyrosinated microtubules modulate mechanotransduction in heart and skeletal muscle. Nat Commun 6:8526
Thandapilly SJ, Louis XL, Yang T, Stringer DM, Yu L, Zhang S, Wigle J, Kardami E, Zahradka P, Taylor C, Anderson HD, Netticadan T (2011) Resveratrol prevents norepinephrine induced hypertrophy in adult rat cardiomyocytes, by activating NO-AMPK pathway. Eur J Pharmacol 668:217–224
Eom GH, Nam YS, Oh JG et al (2014) Regulation of acetylation of histone deacetylase 2 by p300/CBP-associated factor/histone deacetylase 5 in the development of cardiac hypertrophy. Circ Res 114:1133–1143
Sowah D, Brown BF, Quon A, Alvarez BV, Casey JR (2014) Resistance to cardiomyocyte hypertrophy in ae3-/- mice, deficient in the AE3 Cl-/HCO3- exchanger. BMC Cardiovasc Disord 14:89
Miller CL, Oikawa M, Cai Y et al (2009) Role of Ca2+/calmodulin-stimulated cyclic nucleotide phosphodiesterase 1 in mediating cardiomyocyte hypertrophy. Circ Res 105:956–964
Nam YS, Kim Y, Joung H et al (2014) Small heterodimer partner blocks cardiac hypertrophy by interfering with GATA6 signaling. Circ Res 115:493–503
Iribe G, Kohl P (2008) Axial stretch enhances sarcoplasmic reticulum Ca2+ leak and cellular Ca2+ reuptake in Guinea pig ventricular myocytes: experiments and models. Prog Biophys Mol Biol 97:298–311
Prosser BL, Ward CW, Lederer WJ (2013) X-ROS signalling is enhanced and graded by cyclic cardiomyocyte stretch. Cardiovasc Res 98:307–314
Marvin WJ Jr, Robinson RB, Hermsmeyer K (1979) Correlation of function and morphology of neonatal rat and embryonic chick cultured cardiac and vascular muscle cells. Circ Res 45:528–540
Claycomb WC, Palazzo MC (1980) Culture of the terminally differentiated adult cardiac muscle cell: a light and scanning electron microscope study. Dev Biol 80:466–482
Kimes BW, Brandt BL (1976) Properties of a clonal muscle cell line from rat heart. Exp Cell Res 98:367–381
Steinhelper ME, Lanson NA Jr, Dresdner KP et al (1990) Proliferation in vivo and in culture of differentiated adult atrial cardiomyocytes from transgenic mice. Am J Phys 259:H1826–H1834
Delcarpio JB, Lanson NA Jr, Field LJ, Claycomb WC (1991) Morphological characterization of cardiomyocytes isolated from a transplantable cardiac tumor derived from transgenic mouse atria (AT-1 cells). Circ Res 69:1591–1600
Jaffredo T, Chestier A, Bachnou N, Dieterlen-Lievre F (1991) MC29-immortalized clonal avian heart cell lines can partially differentiate in vitro. Exp Cell Res 192:481–491
Claycomb WC, Lanson NA Jr, Stallworth BS et al (1998) HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A 95:2979–2984
Davidson MM, Nesti C, Palenzuela L et al (2005) Novel cell lines derived from adult human ventricular cardiomyocytes. J Mol Cell Cardiol 39:133–147
Menard C, Pupier S, Mornet D, Kitzmann M, Nargeot J, Lory P (1999) Modulation of L-type calcium channel expression during retinoic acid-induced differentiation of H9C2 cardiac cells. J Biol Chem 274:29063–29070
Branco AF, Pereira SP, Gonzalez S, Gusev O, Rizvanov AA, Oliveira PJ (2015) Gene expression profiling of H9c2 myoblast differentiation towards a cardiac-like phenotype. PLoS One 10:e0129303
Watkins SJ, Borthwick GM, Arthur HM (2011) The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. In Vitro Cell Dev Biol Anim 47:125–131
Wang G, Tang C, Yan G, Feng B (2016) Gene expression profiling of H9c2 cells subjected to H2O2-induced apoptosis with/without AF-HF001. Biol Pharm Bull 39:207–214
Kuznetsov AV, Javadov S, Sickinger S, Frotschnig S, Grimm M (1853) H9c2 and HL-1 cells demonstrate distinct features of energy metabolism, mitochondrial function and sensitivity to hypoxia-reoxygenation. Biochim Biophys Acta 2015:276–284
Branco AF, Pereira SL, Moreira AC, Holy J, Sardao VA, Oliveira PJ (2011) Isoproterenol cytotoxicity is dependent on the differentiation state of the cardiomyoblast H9c2 cell line. Cardiovasc Toxicol 11:191–203
McWhinney CD, Hansen C, Robishaw JD (2000) Alpha-1 adrenergic signaling in a cardiac murine atrial myocyte (HL-1) cell line. Mol Cell Biochem 214:111–119
Kitta K, Clement SA, Remeika J, Blumberg JB, Suzuki YJ (2001) Endothelin-1 induces phosphorylation of GATA-4 transcription factor in the HL-1 atrial-muscle cell line. Biochem J 359:375–380
Haas S, Jahnke HG, Moerbt N, von Bergen M, Aharinejad S, Andrukhova O, Robitzki AA (2012) DIGE proteome analysis reveals suitability of ischemic cardiac in vitro model for studying cellular response to acute ischemia and regeneration. PLoS One 7:e31669
Hong JH, Choi JH, Kim TY, Lee KJ (2008) Spiral reentry waves in confluent layer of HL-1 cardiomyocyte cell lines. Biochem Biophys Res Commun 377:1269–1273
Sartiani L, Bochet P, Cerbai E, Mugelli A, Fischmeister R (2002) Functional expression of the hyperpolarization-activated, non-selective cation current I(f) in immortalized HL-1 cardiomyocytes. J Physiol 545:81–92
Dias P, Desplantez T, El-Harasis MA et al (2014) Characterisation of connexin expression and electrophysiological properties in stable clones of the HL-1 myocyte cell line. PLoS One 9:e90266
Fang X, Robinson J, Wang-Hu J, Jiang L, Freeman DA, Rivkees SA, Wendler CC (2015) cAMP induces hypertrophy and alters DNA methylation in HL-1 cardiomyocytes. Am J Physiol Cell Physiol 309:C425–C436
Bloch L, Ndongson-Dongmo B, Kusch A, Dragun D, Heller R, Huber O (2016) Real-time monitoring of hypertrophy in HL-1 cardiomyocytes by impedance measurements reveals different modes of growth. Cytotechnology 68:1897–1907
Asensio-Lopez MC, Soler F, Pascual-Figal D, Fernandez-Belda F, Lax A (2017) Doxorubicin-induced oxidative stress: the protective effect of nicorandil on HL-1 cardiomyocytes. PLoS One 12:e0172803
Dutta D, Xu J, Kim JS, Dunn WA Jr, Leeuwenburgh C (2013) Upregulated autophagy protects cardiomyocytes from oxidative stress-induced toxicity. Autophagy 9:328–344
Truong J, Mailloux RJ, Chan HM (2015) Impact of methylmercury exposure on mitochondrial energetics in AC16 and H9C2 cardiomyocytes. Toxicol in Vitro 29:953–961
Li Q, Qi X, Jia W (2016) 3,3′,5-triiodothyroxine inhibits apoptosis and oxidative stress by the PKM2/PKM1 ratio during oxygen-glucose deprivation/reperfusion AC16 and HCM-a cells: T3 inhibits apoptosis and oxidative stress by PKM2/PKM1 ratio. Biochem Biophys Res Commun 475:51–56
Cui L, Guo J, Zhang Q, Yin J, Li J, Zhou W, Zhang T, Yuan H, Zhao J, Zhang L, Carmichael PL, Peng S (2017) Erythropoietin activates SIRT1 to protect human cardiomyocytes against doxorubicin-induced mitochondrial dysfunction and toxicity. Toxicol Lett 275:28–38
Xiao Y, Yang Z, Wu QQ, Jiang XH, Yuan Y, Chang W, Bian ZY, Zhu JX, Tang QZ (2017) Cucurbitacin B protects against pressure overload induced cardiac hypertrophy. J Cell Biochem 118:3899–3910
Thomson JA, Itskovitz-Eldor J, Shapiro SS et al (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147
Messina E, De Angelis L, Frati G et al (2004) Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res 95:911–921
Goumans MJ, de Boer TP, Smits AM, van Laake L, van Vliet P, Metz CH, Korfage TH, Kats KP, Hochstenbach R, Pasterkamp G, Verhaar MC, van der Heyden M, de Kleijn D, Mummery CL, van Veen T, Sluijter JP, Doevendans PA (2007) TGF-beta1 induces efficient differentiation of human cardiomyocyte progenitor cells into functional cardiomyocytes in vitro. Stem Cell Res 1:138–149
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676
Peters AK, Wouwer GV, Weyn B, Verheyen GR, Vanparys P, Gompel JV (2008) Automated analysis of contractility in the embryonic stem cell test, a novel approach to assess embryotoxicity. Toxicol in Vitro 22:1948–1956
Liu H, Ren C, Liu W, Jiang X, Wang L, Zhu B, Jia W, Lin J, Tan J, Liu X (2017) Embryotoxicity estimation of commonly used compounds with embryonic stem cell test. Mol Med Rep 16:263–271
Burridge PW, Li YF, Matsa E, Wu H, Ong SG, Sharma A, Holmström A, Chang AC, Coronado MJ, Ebert AD, Knowles JW, Telli ML, Witteles RM, Blau HM, Bernstein D, Altman RB, Wu JC (2016) Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat Med 22:547–556
Maillet A, Tan K, Chai X, Sadananda SN, Mehta A, Ooi J, Hayden MR, Pouladi MA, Ghosh S, Shim W, Brunham LR (2016) Modeling doxorubicin-induced cardiotoxicity in human pluripotent stem cell derived-cardiomyocytes. Sci Rep 6:25333
Seewald MJ, Ellinghaus P, Kassner A, Stork I, Barg M, Niebrügge S, Golz S, Summer H, Zweigerdt R, Schräder EM, Feicht S, Jaquet K, Reis S, Körfer R, Milting H (2009) Genomic profiling of developing cardiomyocytes from recombinant murine embryonic stem cells reveals regulation of transcription factor clusters. Physiol Genomics 38:7–15
Shinozawa T, Tsuji A, Imahashi K et al (2009) Gene expression profiling of functional murine embryonic stem cell-derived cardiomyocytes and comparison with adult heart: profiling of murine ESC-derived cardiomyocytes. J Biomol Screen 14:239–245
Lee YK, Ng KM, Lai WH et al (2011) Calcium homeostasis in human induced pluripotent stem cell-derived cardiomyocytes. Stem Cell Rev 7:976–986
Carvajal-Vergara X, Sevilla A, D'Souza SL et al (2010) Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature 465:808–812
Wu H, Lee J, Vincent LG et al (2015) Epigenetic regulation of phosphodiesterases 2A and 3A underlies compromised beta-adrenergic signaling in an iPSC model of dilated cardiomyopathy. Cell Stem Cell 17:89–100
Lin B, Li Y, Han L, Kaplan AD, Ao Y, Kalra S, Bett GCL, Rasmusson RL, Denning C, Yang L (2015) Modeling and study of the mechanism of dilated cardiomyopathy using induced pluripotent stem cells derived from individuals with Duchenne muscular dystrophy. Dis Model Mech 8:457–466
Crombie DE, Curl CL, Raaijmakers AJ et al. Friedreich’s ataxia induced pluripotent stem cell-derived cardiomyocytes display electrophysiological abnormalities and calcium handling deficiency. Aging (Albany NY) 2017;9:1440–1452
Itzhaki I, Maizels L, Huber I et al (2012) Modeling of catecholaminergic polymorphic ventricular tachycardia with patient-specific human-induced pluripotent stem cells. J Am Coll Cardiol 60:990–1000
Guhr A, Kobold S, Seltmann S, Seiler Wulczyn AEM, Kurtz A, Loser P (2018) Recent trends in research with human pluripotent stem cells: impact of research and use of cell lines in experimental research and clinical trials. Stem Cell Reports 11:485–496
Mayourian J, Cashman TJ, Ceholski DK et al (2017) Experimental and computational insight into human mesenchymal stem cell paracrine signaling and heterocellular coupling effects on cardiac contractility and arrhythmogenicity. Circ Res 121:411–423
Grosberg A, Alford PW, McCain ML, Parker KK (2011) Ensembles of engineered cardiac tissues for physiological and pharmacological study: heart on a chip. Lab Chip 11:4165–4173
Lee J, Razu ME, Wang X, Lacerda C, Kim JJ (2015) Biomimetic cardiac microsystems for pathophysiological studies and drug screens. J Lab Autom 20:96–106
Tanaka Y, Sato K, Shimizu T, Yamato M, Okano T, Kitamori T (2007) A micro-spherical heart pump powered by cultured cardiomyocytes. Lab Chip 7:207–212
Li RA, Keung W, Cashman TJ, Backeris PC, Johnson BV, Bardot ES, Wong AOT, Chan PKW, Chan CWY, Costa KD (2018) Bioengineering an electro-mechanically functional miniature ventricular heart chamber from human pluripotent stem cells. Biomaterials 163:116–127
Olejnickova V, Novakova M, Provaznik I (2015) Isolated heart models: cardiovascular system studies and technological advances. Med Biol Eng Comput 53:669–678
de Bakker JM, Coronel R, Tasseron S et al (1990) Ventricular tachycardia in the infarcted, Langendorff-perfused human heart: role of the arrangement of surviving cardiac fibers. J Am Coll Cardiol 15:1594–1607
Akar JG, Akar FG (2006) Mapping arrhythmias in the failing heart: from Langendorff to patient. J Electrocardiol 39:S19–S23
Takewa Y, Chemaly ER, Takaki M, Liang LF, Jin H, Karakikes I, Morel C, Taenaka Y, Tatsumi E, Hajjar RJ (2009) Mechanical work and energetic analysis of eccentric cardiac remodeling in a volume overload heart failure in rats. Am J Physiol Heart Circ Physiol 296:H1117–H1124
Piazza N, Wessells RJ (2011) Drosophila models of cardiac disease. Prog Mol Biol Transl Sci 100:155–210
Zhu XY, Wu SQ, Guo SY, Yang H, Xia B, Li P, Li CQ (2018) A zebrafish heart failure model for assessing therapeutic agents. Zebrafish 15:243–253
Hempel A, Kuhl M (2016) A matter of the heart: the African clawed frog Xenopus as a model for studying vertebrate cardiogenesis and congenital heart defects. J Cardiovasc Dev Dis 3
Mouse Genome Sequencing C, Waterston RH, Lindblad-Toh K et al (2002) Initial sequencing and comparative analysis of the mouse genome. Nature 420:520–562
Flister MJ, Prokop JW, Lazar J, Shimoyama M, Dwinell M, Geurts A, International Committee on Standardized Genetic Nomenclature for Mice., Rat Genome and Nomenclature Committee 2015 Guidelines for establishing genetically modified rat models for cardiovascular research. J Cardiovasc Transl Res 2015;8:269–277
Gehrmann J, Frantz S, Maguire CT, Vargas M, Ducharme A, Wakimoto H, Lee RT, Berul CI (2001) Electrophysiological characterization of murine myocardial ischemia and infarction. Basic Res Cardiol 96:237–250
Redel A, Jazbutyte V, Smul TM et al (2008) Impact of ischemia and reperfusion times on myocardial infarct size in mice in vivo. Exp Biol Med 233:84–93
Christia P, Bujak M, Gonzalez-Quesada C, Chen W, Dobaczewski M, Reddy A, Frangogiannis NG (2013) Systematic characterization of myocardial inflammation, repair, and remodeling in a mouse model of reperfused myocardial infarction. J Histochem Cytochem 61:555–570
Chen J, Ceholski DK, Liang L, Fish K, Hajjar RJ (2017) Variability in coronary artery anatomy affects consistency of cardiac damage after myocardial infarction in mice. Am J Physiol Heart Circ Physiol 313:H275–H282
Lee A, Jeong D, Mitsuyama S et al (2014) The role of SUMO-1 in cardiac oxidative stress and hypertrophy. Antioxid Redox Signal 21:1986–2001
Hampton C, Rosa R, Campbell B, Kennan R, Gichuru L, Ping X, Shen X, Small K, Madwed J, Lynch JJ (2017) Early echocardiographic predictors of outcomes in the mouse transverse aortic constriction heart failure model. J Pharmacol Toxicol Methods 84:93–101
Chaanine AH, Jeong D, Liang L, Chemaly ER, Fish K, Gordon RE, Hajjar RJ (2012) JNK modulates FOXO3a for the expression of the mitochondrial death and mitophagy marker BNIP3 in pathological hypertrophy and in heart failure. Cell Death Dis 3:265
You J, Wu J, Zhang Q et al (2018) Differential cardiac hypertrophy and signaling pathways in pressure versus volume overload. Am J Physiol Heart Circ Physiol 314:H552–H562
Pu M, Gao Z, Zhang X, Liao D, Pu DK, Brennan T, Davidson WR Jr (2009) Impact of mitral regurgitation on left ventricular anatomic and molecular remodeling and systolic function: implication for outcome. Am J Physiol Heart Circ Physiol 296:H1727–H1732
Melenovsky V, Skaroupkova P, Benes J, Torresova V, Kopkan L, Cervenka L (2012) The course of heart failure development and mortality in rats with volume overload due to aorto-caval fistula. Kidney Blood Press Res 35:167–173
Chemaly ER, Kang S, Zhang S, McCollum LT, Chen J, Bénard L, Purushothaman KR, Hajjar RJ, Lebeche D (2013) Differential patterns of replacement and reactive fibrosis in pressure and volume overload are related to the propensity for ischaemia and involve resistin. J Physiol 591:5337–5355
Angsutararux P, Luanpitpong S, Issaragrisil S (2015) Chemotherapy-induced cardiotoxicity: overview of the roles of oxidative stress. Oxidative Med Cell Longev 2015:795602
Muller AM, Fischer A, Katus HA, Kaya Z (2015) Mouse models of autoimmune diseases - autoimmune myocarditis. Curr Pharm Des 21:2498–2512
Pummerer CL, Luze K, Grassl G et al (1996) Identification of cardiac myosin peptides capable of inducing autoimmune myocarditis in BALB/c mice. J Clin Invest 97:2057–2062
Kaya Z, Goser S, Buss SJ et al (2008) Identification of cardiac troponin I sequence motifs leading to heart failure by induction of myocardial inflammation and fibrosis. Circulation 118:2063–2072
Takeshita D, Shimizu J, Kitagawa Y et al (2008) Isoproterenol-induced hypertrophied rat hearts: does short-term treatment correspond to long-term treatment? J Physiol Sci 58:179–188
Wang JJ, Rau C, Avetisyan R et al (2016) Genetic dissection of cardiac remodeling in an isoproterenol-induced heart failure mouse model. PLoS Genet 12:e1006038
Shinohara K, Kishi T, Hirooka Y, Sunagawa K (2015) Circulating angiotensin II deteriorates left ventricular function with sympathoexcitation via brain angiotensin II receptor. Physiol Rep 3
Regan JA, Mauro AG, Carbone S et al (2015) A mouse model of heart failure with preserved ejection fraction due to chronic infusion of a low subpressor dose of angiotensin II. Am J Physiol Heart Circ Physiol 309:H771–H778
Tsukamoto Y, Mano T, Sakata Y, Ohtani T, Takeda Y, Tamaki S, Omori Y, Ikeya Y, Saito Y, Ishii R, Higashimori M, Kaneko M, Miwa T, Yamamoto K, Komuro I (2013) A novel heart failure mice model of hypertensive heart disease by angiotensin II infusion, nephrectomy, and salt loading. Am J Physiol Heart Circ Physiol 305:H1658–H1667
Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M (2010) Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation 121:2747–2754
Lovelock JD, Monasky MM, Jeong EM, Lardin HA, Liu H, Patel BG, Taglieri DM, Gu L, Kumar P, Pokhrel N, Zeng D, Belardinelli L, Sorescu D, Solaro RJ, Dudley SC Jr (2012) Ranolazine improves cardiac diastolic dysfunction through modulation of myofilament calcium sensitivity. Circ Res 110:841–850
Doi R, Masuyama T, Yamamoto K et al (2000) Development of different phenotypes of hypertensive heart failure: systolic versus diastolic failure in Dahl salt-sensitive rats. J Hypertens 18:111–120
Van den Bergh A, Vanderper A, Vangheluwe P et al (2008) Dyslipidaemia in type II diabetic mice does not aggravate contractile impairment but increases ventricular stiffness. Cardiovasc Res 77:371–379
Molinar-Toribio E, Perez-Jimenez J, Ramos-Romero S et al (2014) Cardiovascular disease-related parameters and oxidative stress in SHROB rats, a model for metabolic syndrome. PLoS One 9:e104637
Rosati B, Dong M, Cheng L et al (2008) Evolution of ventricular myocyte electrophysiology. Physiol Genomics 35:262–272
Foster DB, Liu T, Kammers K et al (2016) Integrated omic analysis of a Guinea pig model of heart failure and sudden cardiac death. J Proteome Res 15:3009–3028
Laviolle B, Pape D, Verdier MC, Lavenu A, Bellissant E (2009) Hemodynamic and histomorphometric characteristics of heart failure induced by aortic stenosis in the Guinea pig: comparison of two constriction sizes. Can J Physiol Pharmacol 87:908–914
Fox PR, Basso C, Thiene G, Maron BJ (2014) Spontaneously occurring restrictive nonhypertrophied cardiomyopathy in domestic cats: a new animal model of human disease. Cardiovasc Pathol 23:28–34
Freeman LM, Rush JE, Stern JA, Huggins GS, Maron MS (2017) Feline hypertrophic cardiomyopathy: a spontaneous large animal model of human HCM. Cardiol Res 8:139–142
Suzuki T, Palmer BM, James J et al (2009) Effects of cardiac myosin isoform variation on myofilament function and crossbridge kinetics in transgenic rabbits. Circ Heart Fail 2:334–341
Piacentino V 3rd, Weber CR, Chen X et al (2003) Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res 92:651–658
Edwards AG, Louch WE (2017) Species-dependent mechanisms of cardiac arrhythmia: a cellular focus. Clin Med Insights Cardiol 11:1179546816686061
Freeman GL, Colston JT (1992) Myocardial depression produced by sustained tachycardia in rabbits. Am J Phys 262:H63–H67
Shimizu T, Nakai K, Morimoto Y et al (2009) Simple rabbit model of vulnerable atherosclerotic plaque. Neurol Med Chir (Tokyo) 49:327–332 discussion 332
Thomas SA, Fallavollita JA, Suzuki G, Borgers M, Canty JM Jr (2002) Dissociation of regional adaptations to ischemia and global myolysis in an accelerated swine model of chronic hibernating myocardium. Circ Res 91:970–977
Hedstrom E, Engblom H, Frogner F et al (2009) Infarct evolution in man studied in patients with first-time coronary occlusion in comparison to different species - implications for assessment of myocardial salvage. J Cardiovasc Magn Reson 11:38
Maxwell MP, Hearse DJ, Yellon DM (1987) Species variation in the coronary collateral circulation during regional myocardial ischaemia: a critical determinant of the rate of evolution and extent of myocardial infarction. Cardiovasc Res 21:737–746
Ishikawa K, Aguero J, Tilemann L, Ladage D, Hammoudi N, Kawase Y, Santos-Gallego CG, Fish K, Levine RA, Hajjar RJ (2014) Characterizing preclinical models of ischemic heart failure: differences between LAD and LCx infarctions. Am J Physiol Heart Circ Physiol 307:H1478–H1486
Galvez-Monton C, Prat-Vidal C, Diaz-Guemes I et al (2014) Comparison of two preclinical myocardial infarct models: coronary coil deployment versus surgical ligation. J Transl Med 12:137
Saavedra WF, Tunin RS, Paolocci N et al (2002) Reverse remodeling and enhanced adrenergic reserve from passive external support in experimental dilated heart failure. J Am Coll Cardiol 39:2069–2076
Page BJ, Banas MD, Suzuki G et al (2015) Revascularization of chronic hibernating myocardium stimulates myocyte proliferation and partially reverses chronic adaptations to ischemia. J Am Coll Cardiol 65:684–697
Ishikawa K, Ladage D, Takewa Y et al (2011) Development of a preclinical model of ischemic cardiomyopathy in swine. Am J Physiol Heart Circ Physiol 301:H530–H537
Tuzun E, Oliveira E, Narin C et al (2010) Correlation of ischemic area and coronary flow with ameroid size in a porcine model. J Surg Res 164:38–42
Hinkel R, Howe A, Renner S, Ng J, Lee S, Klett K, Kaczmarek V, Moretti A, Laugwitz KL, Skroblin P, Mayr M, Milting H, Dendorfer A, Reichart B, Wolf E, Kupatt C (2017) Diabetes mellitus-induced microvascular destabilization in the myocardium. J Am Coll Cardiol 69:131–143
Wisenbaugh T, Allen P, Cooper G, Holzgrefe H, Beller G, Carabello B (1983) Contractile function, myosin ATPase activity and isozymes in the hypertrophied pig left ventricle after a chronic progressive pressure overload. Circ Res 53:332–341
Ishikawa K, Aguero J, Oh JG et al (2015) Increased stiffness is the major early abnormality in a pig model of severe aortic stenosis and predisposes to congestive heart failure in the absence of systolic dysfunction. J Am Heart Assoc 4
Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA (2017) Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 136:6–19
Borlaug BA (2014) The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 11:507–515
Zakeri R, Moulay G, Chai Q, Ogut O, Hussain S, Takahama H, Lu T, Wang XL, Linke WA, Lee HC, Redfield MM (2016) Left atrial remodeling and atrioventricular coupling in a canine model of early heart failure with preserved ejection fraction. Circ Heart Fail 9
Munagala VK, Hart CY, Burnett JC Jr, Meyer DM, Redfield MM (2005) Ventricular structure and function in aged dogs with renal hypertension: a model of experimental diastolic heart failure. Circulation 111:1128–1135
Schwarzl M, Hamdani N, Seiler S, Alogna A, Manninger M, Reilly S, Zirngast B, Kirsch A, Steendijk P, Verderber J, Zweiker D, Eller P, Höfler G, Schauer S, Eller K, Maechler H, Pieske BM, Linke WA, Casadei B, Post H (2015) A porcine model of hypertensive cardiomyopathy: implications for heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol 309:H1407–H1418
Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA (2006) Controversies in ventricular remodelling. Lancet 367:356–367
Leroux AA, Moonen ML, Pierard LA, Kolh P, Amory H (2012) Animal models of mitral regurgitation induced by mitral valve chordae tendineae rupture. J Heart Valve Dis 21:416–423
Watanabe S, Ishikawa K, Fish K et al (2017) Protein phosphatase inhibitor-1 gene therapy in a swine model of nonischemic heart failure. J Am Coll Cardiol 70:1744–1756
Beeri R, Yosefy C, Guerrero JL, Nesta F, Abedat S, Chaput M, del Monte F, Handschumacher MD, Stroud R, Sullivan S, Pugatsch T, Gilon D, Vlahakes GJ, Spinale FG, Hajjar RJ, Levine RA (2008) Mitral regurgitation augments post-myocardial infarction remodeling failure of hypertrophic compensation. J Am Coll Cardiol 51:476–486
Naito N, Nishimura T, Takewa Y et al (2016) What is the optimal setting for a continuous-flow left ventricular assist device in severe mitral regurgitation? Artif Organs 40:1039–1045
Chaput M, Handschumacher MD, Guerrero JL, Holmvang G, Dal-Bianco JP, Sullivan S, Vlahakes GJ, Hung J, Levine RA, for the Leducq Foundation MITRAL Transatlantic Network (2009) Mitral leaflet adaptation to ventricular remodeling: prospective changes in a model of ischemic mitral regurgitation. Circulation 120:S99–S103
Ishikawa K, Watanabe S, Hammoudi N et al (2018) Reduced longitudinal contraction is associated with ischemic mitral regurgitation after posterior MI. Am J Physiol Heart Circ Physiol 314:H322–H329
Lu X, Zhang ZD, Guo X, Choy JS, Yang J, Svendsen M, Kassab G (2014) Response of various conduit arteries in tachycardia- and volume overload-induced heart failure. PLoS One 9:e101645
Woitek F, Zentilin L, Hoffman NE, Powers JC, Ottiger I, Parikh S, Kulczycki AM, Hurst M, Ring N, Wang T, Shaikh F, Gross P, Singh H, Kolpakov MA, Linke A, Houser SR, Rizzo V, Sabri A, Madesh M, Giacca M, Recchia FA (2015) Intracoronary cytoprotective gene therapy: a study of VEGF-B167 in a pre-clinical animal model of dilated cardiomyopathy. J Am Coll Cardiol 66:139–153
Spinale FG, Coker ML, Thomas CV, Walker JD, Mukherjee R, Hebbar L (1998) Time-dependent changes in matrix metalloproteinase activity and expression during the progression of congestive heart failure: relation to ventricular and myocyte function. Circ Res 82:482–495
Shinbane JS, Wood MA, Jensen DN, Ellenbogen KA, Fitzpatrick AP, Scheinman MM (1997) Tachycardia-induced cardiomyopathy: a review of animal models and clinical studies. J Am Coll Cardiol 29:709–715
Riegger AJ, Liebau G (1982) The renin-angiotensin-aldosterone system, antidiuretic hormone and sympathetic nerve activity in an experimental model of congestive heart failure in the dog. Clin Sci (Lond) 62:465–469
Monnet E, Orton EC (1999) A canine model of heart failure by intracoronary adriamycin injection: hemodynamic and energetic results. J Card Fail 5:255–264
Toyoda Y, Okada M, Kashem MA (1998) A canine model of dilated cardiomyopathy induced by repetitive intracoronary doxorubicin administration. J Thorac Cardiovasc Surg 115:1367–1373
Hyldebrandt JA, Siven E, Agger P et al (2015) Effects of milrinone and epinephrine or dopamine on biventricular function and hemodynamics in an animal model with right ventricular failure after pulmonary artery banding. Am J Physiol Heart Circ Physiol 309:H206–H212
Aguero J, Ishikawa K, Hadri L, Santos-Gallego C, Fish K, Hammoudi N, Chaanine A, Torquato S, Naim C, Ibanez B, Pereda D, García-Alvarez A, Fuster V, Sengupta PP, Leopold JA, Hajjar RJ (2014) Characterization of right ventricular remodeling and failure in a chronic pulmonary hypertension model. Am J Physiol Heart Circ Physiol 307:H1204–H1215
van Duin RWB, Stam K, Cai Z et al (2018) Transition from post-capillary pulmonary hypertension to combined pre- and post-capillary pulmonary hypertension in swine: a key role for endothelin. J Physiol
Aguero J, Ishikawa K, Fish KM, Hammoudi N, Hadri L, Garcia-Alvarez A, Ibanez B, Fuster V, Hajjar RJ, Leopold JA (2015) Combination proximal pulmonary artery coiling and distal embolization induces chronic elevations in pulmonary artery pressure in swine. PLoS One 10:e0124526
Pereda D, Garcia-Lunar I, Sierra F et al (2016) Magnetic resonance characterization of cardiac adaptation and myocardial fibrosis in pulmonary hypertension secondary to systemic-to-pulmonary shunt. Circ Cardiovasc Imaging 9
Sage E, Mercier O, Herve P et al (2012) Right lung ischemia induces contralateral pulmonary vasculopathy in an animal model. J Thorac Cardiovasc Surg 143:967–973
Funding
This work is supported by AHA-SDG 17SDG33410873 (K.I.), and AHA 17POST33410877 (J.G.O.), NIH R01 HL139963 (K.I.), AHA-18TPA34170460 (C.K.), R00 HL116645 (C.K.), R01 HL119046, R01 HL117505, R01 HL128099, R01 HL129814, R01HL131404, & T32 HL007824 (R. J. H.), and a Transatlantic Leducq Foundation grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oh, J.G., Kho, C., Hajjar, R.J. et al. Experimental models of cardiac physiology and pathology. Heart Fail Rev 24, 601–615 (2019). https://doi.org/10.1007/s10741-019-09769-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-019-09769-2