Abstract
Heart failure (HF) is a common cardiac syndrome, whose pathophysiology involves complex mechanisms, some of which remain unknown. Diabetes mellitus (DM) constitutes not only a glucose metabolic disorder accompanied by insulin resistance but also a risk factor for cardiovascular disease and HF. During the last years though emerging data set up, a bidirectional interrelationship between these two entities. In the case of DM impaired calcium homeostasis, free fatty acid metabolism, redox state, and advance glycation end products may accelerate cardiac dysfunction. On the other hand, when HF exists, hypoperfusion of the liver and pancreas, b-blocker and diuretic treatment, and autonomic nervous system dysfunction may cause impairment of glucose metabolism. These molecular pathways may be used as therapeutic targets for novel antidiabetic agents. Peroxisome proliferator-activated receptors (PPARs) not only improve insulin resistance and glucose and lipid metabolism but also manifest a diversity of actions directly or indirectly associated with systolic or diastolic performance of left ventricle and symptoms of HF. Interestingly, they may beneficially affect remodeling of the left ventricle, fibrosis, and diastolic performance but they may cause impaired water handing, sodium retention, and decompensation of HF which should be taken into consideration in the management of patients with DM. In this review article, we present the pathophysiological data linking HF with DM and we focus on the molecular mechanisms of PPARs agonists in left ventricle systolic and diastolic performance providing useful insights in the molecular mechanism of this class of metabolically active regiments.


Similar content being viewed by others
References
Economou EK, Oikonomou E, Siasos G, Papageorgiou N, Tsalamandris S, Mourouzis K, Papaioanou S, Tousoulis D (2015) The role of microRNAs in coronary artery disease: from pathophysiology to diagnosis and treatment. Atherosclerosis 241:624–633. https://doi.org/10.1016/j.atherosclerosis.2015.06.037
Siasos G, Tousoulis D, Tourikis P, Mazaris S, Zakynthinos G, Oikonomou E, Kokkou E, Kollia C, Stefanadis C (2013) MicroRNAs in cardiovascular therapeutics. Curr Top Med Chem 13:1605–1618
Duan SZ, Ivashchenko CY, Usher MG, Mortensen RM (2008) PPAR-gamma in the cardiovascular system. PPAR Res 2008:745804. https://doi.org/10.1155/2008/745804
Kahn R, Buse J, Ferrannini E, Stern M, American Diabetes A, European Association for the Study of D (2005) The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 28:2289–2304
DeFronzo RA, Ferrannini E (1991) Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 14:173–194
Kaplan NM (1989) The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch Intern Med 149:1514–1520
Gu K, Cowie CC, Harris MI (1999) Diabetes and decline in heart disease mortality in US adults. JAMA 281:1291–1297
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL (2013) 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128:1810–1852. https://doi.org/10.1161/CIR.0b013e31829e8807
FDA guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071627.pdf. Accessed 2 Aug 2017
Kannel WB, Hjortland M, Castelli WP (1974) Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 34:29–34
Greenberg BH, Abraham WT, Albert NM, Chiswell K, Clare R, Stough WG, Gheorghiade M, O'Connor CM, Sun JL, Yancy CW, Young JB, Fonarow GC (2007) Influence of diabetes on characteristics and outcomes in patients hospitalized with heart failure: a report from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am Heart J 154(277):e271–e278. https://doi.org/10.1016/j.ahj.2007.05.001
Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB (2004) The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care 27:1879–1884
Cavender MA, Steg PG, Smith SC Jr, Eagle K, Ohman EM, Goto S, Kuder J, Im K, Wilson PW, Bhatt DL, Investigators RR (2015) Impact of diabetes mellitus on hospitalization for heart failure, cardiovascular events, and death: outcomes at 4 years from the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Circulation 132:923–931. https://doi.org/10.1161/CIRCULATIONAHA.114.014796
Dei Cas A, Khan SS, Butler J, Mentz RJ, Bonow RO, Avogaro A, Tschoepe D, Doehner W, Greene SJ, Senni M, Gheorghiade M, Fonarow GC (2015) Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail 3:136–145. https://doi.org/10.1016/j.jchf.2014.08.004
Wong YW, Thomas L, Sun JL, McMurray JJ, Krum H, Hernandez AF, Rutten GE, Leiter LA, Standl E, Haffner SM, Mazzone T, Martinez FA, Tognoni G, Giles T, Califf RM (2013) Predictors of incident heart failure hospitalizations among patients with impaired glucose tolerance: insight from the Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research study. Circ Heart Fail 6:203–210. https://doi.org/10.1161/CIRCHEARTFAILURE.112.000086
Iribarren C, Karter AJ, Go AS, Ferrara A, Liu JY, Sidney S, Selby JV (2001) Glycemic control and heart failure among adult patients with diabetes. Circulation 103:2668–2673
Gilca GE, Stefanescu G, Badulescu O, Tanase DM, Bararu I, Ciocoiu M (2017) Diabetic cardiomyopathy: current approach and potential diagnostic and therapeutic targets. J Diabetes Res 2017:1310265. https://doi.org/10.1155/2017/1310265
Holscher ME, Bode C, Bugger H (2016) Diabetic cardiomyopathy: does the type of diabetes matter? Int J Mol Sci 17. https://doi.org/10.3390/ijms17122136
Sarma S, Mentz RJ, Kwasny MJ, Fought AJ, Huffman M, Subacius H, Nodari S, Konstam M, Swedberg K, Maggioni AP, Zannad F, Bonow RO, Gheorghiade M, investigators E (2013) Association between diabetes mellitus and post-discharge outcomes in patients hospitalized with heart failure: findings from the EVEREST trial. Eur J Heart Fail 15:194–202. https://doi.org/10.1093/eurjhf/hfs153
Ikeda Y, Inomata T, Fujita T, Iida Y, Kaida T, Nabeta T, Ishii S, Maekawa E, Yanagisawa T, Mizutani T, Naruke T, Koitabashi T, Takeuchi I, Ako J (2017) Higher hemoglobin A1c levels are associated with impaired left ventricular diastolic function and higher incidence of adverse cardiac events in patients with nonischemic dilated cardiomyopathy. Heart Vessel 32:446–457. https://doi.org/10.1007/s00380-016-0895-x
Maiello M, Zito A, Cecere A, Ciccone MM, Palmiero P (2017) Left ventricular diastolic dysfunction in normotensive postmenopausal women with type 2 diabetes mellitus. Cardiol J 24:51–56. https://doi.org/10.5603/CJ.a2016.0064
Dauriz M, Targher G, Laroche C, Temporelli PL, Ferrari R, Anker S, Coats A, Filippatos G, Crespo-Leiro M, Mebazaa A, Piepoli MF, Maggioni AP, Tavazzi L, Registry E-HHFL-T (2017) Association between diabetes and 1-year adverse clinical outcomes in a multinational cohort of ambulatory patients with chronic heart failure: Results from the ESC-HFA Heart Failure Long-Term Registry. Diabetes Care 40:671–678. https://doi.org/10.2337/dc16-2016
Lind M, Olsson M, Rosengren A, Svensson AM, Bounias I, Gudbjornsdottir S (2012) The relationship between glycaemic control and heart failure in 83,021 patients with type 2 diabetes. Diabetologia 55:2946–2953. https://doi.org/10.1007/s00125-012-2681-3
Cunha FM, Marques P, Pereira J, Pinto MJ, Rodrigues P, Moreira H, Lourenco P, Bettencourt P (2016) Insulin treatment may not be associated with worse prognosis in acute heart failure diabetic patients. Minerva Endocrinol 42(4):318–324. https://doi.org/10.23736/S0391-1977.16.02535-9
Glogner S, Rosengren A, Olsson M, Gudbjornsdottir S, Svensson AM, Lind M (2014) The association between BMI and hospitalization for heart failure in 83,021 persons with type 2 diabetes: a population-based study from the Swedish National Diabetes Registry. Diabet Med 31:586–594. https://doi.org/10.1111/dme.12340
Echouffo-Tcheugui JB, Xu H, DeVore AD, Schulte PJ, Butler J, Yancy CW, Bhatt DL, Hernandez AF, Heidenreich PA, Fonarow GC (2016) Temporal trends and factors associated with diabetes mellitus among patients hospitalized with heart failure: findings from Get with the Guidelines-Heart Failure registry. Am Heart J 182:9–20. https://doi.org/10.1016/j.ahj.2016.07.025
Seferovic PM, Paulus WJ (2015) Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. Eur Heart J 36:1718–1727, 1727a-1727c. https://doi.org/10.1093/eurheartj/ehv134
Ares-Carrasco S, Picatoste B, Camafeita E, Carrasco-Navarro S, Zubiri I, Ortiz A, Egido J, Lopez JA, Tunon J, Lorenzo O (2012) Proteome changes in the myocardium of experimental chronic diabetes and hypertension: role of PPARalpha in the associated hypertrophy. J Proteome 75:1816–1829. https://doi.org/10.1016/j.jprot.2011.12.023
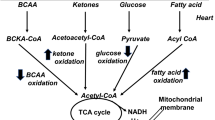
Fukushima A, Lopaschuk GD (2016) Acetylation control of cardiac fatty acid beta-oxidation and energy metabolism in obesity, diabetes, and heart failure. Biochim Biophys Acta 1862:2211–2220. https://doi.org/10.1016/j.bbadis.2016.07.020
Pallares-Mendez R, Aguilar-Salinas CA, Cruz-Bautista I, Del Bosque-Plata L (2016) Metabolomics in diabetes, a review. Ann Med 48:89–102. https://doi.org/10.3109/07853890.2015.1137630
Li C, Lu C, Zhao X, Chen X (2015) Comparison between myocardial infarction and diabetes mellitus damage caused angiogenesis or energy metabolism. Int J Clin Exp Med 8:22371–22376
Kim G, Jo K, Kim KJ, Lee YH, Han E, Yoon HJ, Wang HJ, Kang ES, Yun M (2015) Visceral adiposity is associated with altered myocardial glucose uptake measured by (18)FDG-PET in 346 subjects with normal glucose tolerance, prediabetes, and type 2 diabetes. Cardiovasc Diabetol 14:148. https://doi.org/10.1186/s12933-015-0310-4
Murray AJ, Cole MA, Lygate CA, Carr CA, Stuckey DJ, Little SE, Neubauer S, Clarke K (2008) Increased mitochondrial uncoupling proteins, respiratory uncoupling and decreased efficiency in the chronically infarcted rat heart. J Mol Cell Cardiol 44:694–700. https://doi.org/10.1016/j.yjmcc.2008.01.008
Murray AJ, Anderson RE, Watson GC, Radda GK, Clarke K (2004) Uncoupling proteins in human heart. Lancet 364:1786–1788. https://doi.org/10.1016/S0140-6736(04)17402-3
Winhofer Y, Krssak M, Wolf P, Anderwald CH, Geroldinger A, Heinze G, Baumgartner-Parzer S, Marculescu R, Stulnig T, Wolzt M, Trattnig S, Luger A, Krebs M (2015) Free fatty acid availability is closely related to myocardial lipid storage and cardiac function in hypoglycemia counterregulation. Am J Phys Endocrinol Metab 308:E631–E640. https://doi.org/10.1152/ajpendo.00371.2014
Mori J, Alrob OA, Wagg CS, Harris RA, Lopaschuk GD, Oudit GY (2013) ANG II causes insulin resistance and induces cardiac metabolic switch and inefficiency: a critical role of PDK4. Am J Phys Heart Circ Phys 304:H1103–H1113. https://doi.org/10.1152/ajpheart.00636.2012
Zhao G, Jeoung NH, Burgess SC, Rosaaen-Stowe KA, Inagaki T, Latif S, Shelton JM, McAnally J, Bassel-Duby R, Harris RA, Richardson JA, Kliewer SA (2008) Overexpression of pyruvate dehydrogenase kinase 4 in heart perturbs metabolism and exacerbates calcineurin-induced cardiomyopathy. Am J Phys Heart Circ Phys 294:H936–H943. https://doi.org/10.1152/ajpheart.00870.2007
Ishimura S, Furuhashi M, Watanabe Y, Hoshina K, Fuseya T, Mita T, Okazaki Y, Koyama M, Tanaka M, Akasaka H, Ohnishi H, Yoshida H, Saitoh S, Miura T (2013) Circulating levels of fatty acid-binding protein family and metabolic phenotype in the general population. PLoS One 8:e81318. https://doi.org/10.1371/journal.pone.0081318
Cabre A, Valdovinos P, Lazaro I, Bonet G, Bardaji A, Masana L (2013) Parallel evolution of circulating FABP4 and NT-proBNP in heart failure patients. Cardiovasc Diabetol 12:72. https://doi.org/10.1186/1475-2840-12-72
Mazovets OL, Trifonov IR, Katrukha AG, Bereznikova AV, Medvedeva MV, Deev AD, Gratsianksii NA (2008) Heart fatty acid binding protein in patients hospitalized because of worsening heart failure. Relation to prognosis of death. Kardiologiia 48:24–29
Sun X, Pan H, Tan H, Yu Y (2012) High free fatty acids level related with cardiac dysfunction in obese rats. Diabetes Res Clin Pract 95:251–259. https://doi.org/10.1016/j.diabres.2011.10.028
Zhang Y, Ling Y, Yang L, Cheng Y, Yang P, Song X, Tang H, Zhong Y, Tang L, He S, Yang S, Chen A, Wang X (2017) Liraglutide relieves myocardial damage by promoting autophagy via AMPK-mTOR signaling pathway in zucker diabetic fatty rat. Mol Cell Endocrinol 448:98–107. https://doi.org/10.1016/j.mce.2017.03.029
Son NH, Ananthakrishnan R, Yu S, Khan RS, Jiang H, Ji R, Akashi H, Li Q, O'Shea K, Homma S, Goldberg IJ, Ramasamy R (2012) Cardiomyocyte aldose reductase causes heart failure and impairs recovery from ischemia. PLoS One 7:e46549. https://doi.org/10.1371/journal.pone.0046549
Ussher JR, Folmes CD, Keung W, Fillmore N, Jaswal JS, Cadete VJ, Beker DL, Lam VH, Zhang L, Lopaschuk GD (2012) Inhibition of serine palmitoyl transferase I reduces cardiac ceramide levels and increases glycolysis rates following diet-induced insulin resistance. PLoS One 7:e37703. https://doi.org/10.1371/journal.pone.0037703
Ramirez E, Klett-Mingo M, Ares-Carrasco S, Picatoste B, Ferrarini A, Ruperez FJ, Caro-Vadillo A, Barbas C, Egido J, Tunon J, Lorenzo O (2013) Eplerenone attenuated cardiac steatosis, apoptosis and diastolic dysfunction in experimental type-II diabetes. Cardiovasc Diabetol 12:172. https://doi.org/10.1186/1475-2840-12-172
Knowles CJ, Cebova M, Pinz IM (2013) Palmitate diet-induced loss of cardiac caveolin-3: a novel mechanism for lipid-induced contractile dysfunction. PLoS One 8:e61369. https://doi.org/10.1371/journal.pone.0061369
Artwohl M, Lindenmair A, Roden M, Waldhausl WK, Freudenthaler A, Klosner G, Ilhan A, Luger A, Baumgartner-Parzer SM (2009) Fatty acids induce apoptosis in human smooth muscle cells depending on chain length, saturation, and duration of exposure. Atherosclerosis 202:351–362. https://doi.org/10.1016/j.atherosclerosis.2008.05.030
Jensen MK, Bartz TM, Mukamal KJ, Djousse L, Kizer JR, Tracy RP, Zieman SJ, Rimm EB, Siscovick DS, Shlipak M, Ix JH (2013) Fetuin-A, type 2 diabetes, and risk of cardiovascular disease in older adults: the cardiovascular health study. Diabetes Care 36:1222–1228. https://doi.org/10.2337/dc12-1591
Chang WT, Tsai WC, Wu CH, Lee YW, Tai YL, Li YH, Tsai LM, Chen JH, Liu PY (2015) Fetuin-A as a predicator of sarcopenic left ventricular dysfunction. Sci Rep 5:12078. https://doi.org/10.1038/srep12078
Cohen K, Waldman M, Abraham NG, Laniado-Schwartzman M, Gurfield D, Aravot D, Arad M, Hochhauser E (2017) Caloric restriction ameliorates cardiomyopathy in animal model of diabetes. Exp Cell Res 350:147–153. https://doi.org/10.1016/j.yexcr.2016.11.016
Avgerinou G, Tousoulis D, Siasos G, Oikonomou E, Maniatis K, Papageorgiou N, Paraskevopoulos T, Miliou A, Koumaki D, Latsios G, Therianiou A, Trikas A, Kampoli AM, Stefanadis C (2011) Anti-tumor necrosis factor alpha treatment with adalimumab improves significantly endothelial function and decreases inflammatory process in patients with chronic psoriasis. Int J Cardiol 151:382–383. https://doi.org/10.1016/j.ijcard.2011.06.112
Alvarez-Guardia D, Palomer X, Coll T, Serrano L, Rodriguez-Calvo R, Davidson MM, Merlos M, El Kochairi I, Michalik L, Wahli W, Vazquez-Carrera M (2011) PPARbeta/delta activation blocks lipid-induced inflammatory pathways in mouse heart and human cardiac cells. Biochim Biophys Acta 1811:59–67. https://doi.org/10.1016/j.bbalip.2010.11.002
Neschen S, Morino K, Dong J, Wang-Fischer Y, Cline GW, Romanelli AJ, Rossbacher JC, Moore IK, Regittnig W, Munoz DS, Kim JH, Shulman GI (2007) N-3 fatty acids preserve insulin sensitivity in vivo in a peroxisome proliferator-activated receptor-alpha-dependent manner. Diabetes 56:1034–1041. https://doi.org/10.2337/db06-1206
Gilde AJ, van der Lee KA, Willemsen PH, Chinetti G, van der Leij FR, van der Vusse GJ, Staels B, van Bilsen M (2003) Peroxisome proliferator-activated receptor (PPAR) alpha and PPARbeta/delta, but not PPARgamma, modulate the expression of genes involved in cardiac lipid metabolism. Circ Res 92:518–524. https://doi.org/10.1161/01.RES.0000060700.55247.7C
Huss JM, Kelly DP (2004) Nuclear receptor signaling and cardiac energetics. Circ Res 95:568–578. https://doi.org/10.1161/01.RES.0000141774.29937.e3
Palomer X, Capdevila-Busquets E, Garreta G, Davidson MM, Vazquez-Carrera M (2014) PPARalpha attenuates palmitate-induced endoplasmic reticulum stress in human cardiac cells by enhancing AMPK activity. Clin Investig Arterioscler 26:255–267. https://doi.org/10.1016/j.arteri.2014.02.003
Linz W, Wohlfart P, Baader M, Breitschopf K, Falk E, Schafer HL, Gerl M, Kramer W, Rutten H (2009) The peroxisome proliferator-activated receptor-alpha (PPAR-alpha) agonist, AVE8134, attenuates the progression of heart failure and increases survival in rats. Acta Pharmacol Sin 30:935–946. https://doi.org/10.1038/aps.2009.58
Burkart EM, Sambandam N, Han X, Gross RW, Courtois M, Gierasch CM, Shoghi K, Welch MJ, Kelly DP (2007) Nuclear receptors PPARbeta/delta and PPARalpha direct distinct metabolic regulatory programs in the mouse heart. J Clin Invest 117:3930–3939. https://doi.org/10.1172/JCI32578
Wang XJ, Zhang J, Wang SQ, Xu WR, Cheng XC, Wang RL (2014) Identification of novel multitargeted PPARalpha/gamma/delta pan agonists by core hopping of rosiglitazone. Drug Des Devel Ther 8:2255–2262. https://doi.org/10.2147/DDDT.S70383
Czarnowska E, Turska-Kmiec A (2012) Current opinions on mechanisms of energetic abnormalities in heart. Significance of the PPARalpha expression and therapeutic objects. Kardiol Pol 70:1061–1067
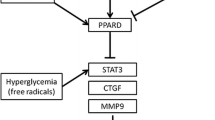
Koulis C, Watson AM, Gray SP, Jandeleit-Dahm KA (2015) Linking RAGE and Nox in diabetic micro- and macrovascular complications. Diabetes Metab 41:272–281. https://doi.org/10.1016/j.diabet.2015.01.006
Brings S, Fleming T, Freichel M, Muckenthaler MU, Herzig S, Nawroth PP (2017) Dicarbonyls and advanced glycation end-products in the development of diabetic complications and targets for intervention. Int J Mol Sci 18. https://doi.org/10.3390/ijms18050984
Yamazaki KG, Gonzalez E, Zambon AC (2012) Crosstalk between the renin-angiotensin system and the advance glycation end product axis in the heart: role of the cardiac fibroblast. J Cardiovasc Transl Res 5:805–813. https://doi.org/10.1007/s12265-012-9405-4
Kumari K, Sahib MK (1993) Susceptibility of different rat tissues to non-enzymatic protein glycosylation in experimental diabetes. Indian J Exp Biol 31:194–195
Nozynski J, Zakliczynski M, Konecka-Mrowka D, Zielinska T, Zakliczynska H, Nikiel B, Mlynarczyk-Liszka J, Mrowka A, Zembala-Nozynska E, Pijet M, Rdzanowska K, Lange D, Przybylski R, Zembala M (2012) Advanced glycation end product accumulation in the cardiomyocytes of heart failure patients with and without diabetes. Ann Transplant 17:53–61
Kranstuber AL, Del Rio C, Biesiadecki BJ, Hamlin RL, Ottobre J, Gyorke S, Lacombe VA (2012) Advanced glycation end product cross-link breaker attenuates diabetes-induced cardiac dysfunction by improving sarcoplasmic reticulum calcium handling. Front Physiol 3:292. https://doi.org/10.3389/fphys.2012.00292
Kang S, Dahl R, Hsieh W, Shin A, Zsebo KM, Buettner C, Hajjar RJ, Lebeche D (2016) Small molecular allosteric activator of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) attenuates diabetes and metabolic disorders. J Biol Chem 291:5185–5198. https://doi.org/10.1074/jbc.M115.705012
Nozynski J, Zakliczynski M, Konecka-Mrowka D, Zakliczynska H, Pijet M, Zembala-Nozynska E, Lange D, Zembala M (2013) Advanced glycation end products and lipofuscin deposits share the same location in cardiocytes of the failing heart. Exp Gerontol 48:223–228. https://doi.org/10.1016/j.exger.2012.09.002
Kass DA, Shapiro EP, Kawaguchi M, Capriotti AR, Scuteri A, deGroof RC, Lakatta EG (2001) Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation 104:1464–1470
Tanaka S, Avigad G, Brodsky B, Eikenberry EF (1988) Glycation induces expansion of the molecular packing of collagen. J Mol Biol 203:495–505
Toma L, Sanda GM, Deleanu M, Stancu CS, Sima AV (2016) Glycated LDL increase VCAM-1 expression and secretion in endothelial cells and promote monocyte adhesion through mechanisms involving endoplasmic reticulum stress. Mol Cell Biochem 417:169–179. https://doi.org/10.1007/s11010-016-2724-z
Mohanan Nair M, Zhao R, Xie X, Shen GX (2016) Impact of glycated LDL on endothelial nitric oxide synthase in vascular endothelial cells: involvement of transmembrane signaling and endoplasmic reticulum stress. J Diabetes Complicat 30:391–397. https://doi.org/10.1016/j.jdiacomp.2016.01.008
Garcia R, Merino D, Gomez JM, Nistal JF, Hurle MA, Cortajarena AL, Villar AV (2016) Extracellular heat shock protein 90 binding to TGFbeta receptor I participates in TGFbeta-mediated collagen production in myocardial fibroblasts. Cell Signal 28:1563–1579. https://doi.org/10.1016/j.cellsig.2016.07.003
Striker LJ, Striker GE (1996) Administration of AGEs in vivo induces extracellular matrix gene expression. Nephrol Dial Transplant 11(Suppl 5):62–65
Ko SY, Lin IH, Shieh TM, Ko HA, Chen HI, Chi TC, Chang SS, Hsu YC (2013) Cell hypertrophy and MEK/ERK phosphorylation are regulated by glyceraldehyde-derived AGEs in cardiomyocyte H9c2 cells. Cell Biochem Biophys 66:537–544. https://doi.org/10.1007/s12013-012-9501-8
Zhang B, Shen Q, Chen Y, Pan R, Kuang S, Liu G, Sun G, Sun X (2017) Myricitrin alleviates oxidative stress-induced inflammation and apoptosis and protects mice against diabetic cardiomyopathy. Sci Rep 7:44239. https://doi.org/10.1038/srep44239
Campbell DJ, Somaratne JB, Jenkins AJ, Prior DL, Yii M, Kenny JF, Newcomb AE, Schalkwijk CG, Black MJ, Kelly DJ (2012) Diastolic dysfunction of aging is independent of myocardial structure but associated with plasma advanced glycation end-product levels. PLoS One 7:e49813. https://doi.org/10.1371/journal.pone.0049813
Willemsen S, Hartog JW, Hummel YM, van Ruijven MH, van der Horst IC, van Veldhuisen DJ, Voors AA (2011) Tissue advanced glycation end products are associated with diastolic function and aerobic exercise capacity in diabetic heart failure patients. Eur J Heart Fail 13:76–82. https://doi.org/10.1093/eurjhf/hfq168
Hartog JW, Willemsen S, van Veldhuisen DJ, Posma JL, van Wijk LM, Hummel YM, Hillege HL, Voors AA, investigators B (2011) Effects of alagebrium, an advanced glycation endproduct breaker, on exercise tolerance and cardiac function in patients with chronic heart failure. Eur J Heart Fail 13:899–908. https://doi.org/10.1093/eurjhf/hfr067
Wang LJ, Lu L, Zhang FR, Chen QJ, De Caterina R, Shen WF (2011) Increased serum high-mobility group box-1 and cleaved receptor for advanced glycation endproducts levels and decreased endogenous secretory receptor for advanced glycation endproducts levels in diabetic and non-diabetic patients with heart failure. Eur J Heart Fail 13:440–449. https://doi.org/10.1093/eurjhf/hfq231
Volz HC, Seidel C, Laohachewin D, Kaya Z, Muller OJ, Pleger ST, Lasitschka F, Bianchi ME, Remppis A, Bierhaus A, Katus HA, Andrassy M (2010) HMGB1: the missing link between diabetes mellitus and heart failure. Basic Res Cardiol 105:805–820. https://doi.org/10.1007/s00395-010-0114-3
Huang Q, Yang Z, Zhou JP, Luo Y (2017) HMGB1 induces endothelial progenitor cells apoptosis via RAGE-dependent PERK/eIF2alpha pathway. Mol Cell Biochem. https://doi.org/10.1007/s11010-017-2976-2
Lin H, Shen L, Zhang X, Xie J, Hao H, Zhang Y, Chen Z, Yamamoto H, Liao W, Bin J, Cao S, Huang X, Liao Y (2016) HMGB1-RAGE axis makes no contribution to cardiac remodeling induced by pressure-overload. PLoS One 11:e0158514. https://doi.org/10.1371/journal.pone.0158514
Davlouros PA, Gkizas V, Vogiatzi C, Giannopoulos G, Alexopoulos D, Deftereos S (2016) Calcium homeostasis and kinetics in heart failure. Med Chem 12:151–161
Hohendanner F, Ljubojevic S, MacQuaide N, Sacherer M, Sedej S, Biesmans L, Wakula P, Platzer D, Sokolow S, Herchuelz A, Antoons G, Sipido K, Pieske B, Heinzel FR (2013) Intracellular dyssynchrony of diastolic cytosolic [Ca(2)(+)] decay in ventricular cardiomyocytes in cardiac remodeling and human heart failure. Circ Res 113:527–538. https://doi.org/10.1161/CIRCRESAHA.113.300895
Nassal DM, Wan X, Liu H, Laurita KR, Deschenes I (2017) KChIP2 regulates the cardiac Ca2+ transient and myocyte contractility by targeting ryanodine receptor activity. PLoS One 12:e0175221. https://doi.org/10.1371/journal.pone.0175221
Shanks J, Herring N, Johnson E, Liu K, Li D, Paterson DJ (2017) Overexpression of sarcoendoplasmic reticulum calcium ATPase 2a promotes cardiac sympathetic neurotransmission via abnormal endoplasmic reticulum and mitochondria Ca2+ regulation. Hypertension 69:625–632. https://doi.org/10.1161/HYPERTENSIONAHA.116.08507
Pan X, Liu J, Nguyen T, Liu C, Sun J, Teng Y, Fergusson MM, Rovira II, Allen M, Springer DA, Aponte AM, Gucek M, Balaban RS, Murphy E, Finkel T (2013) The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol 15:1464–1472. https://doi.org/10.1038/ncb2868
Okatan EN, Durak AT, Turan B (2016) Electrophysiological basis of metabolic-syndrome-induced cardiac dysfunction. Can J Physiol Pharmacol 94:1064–1073. https://doi.org/10.1139/cjpp-2015-0531
Yan D, Luo X, Li Y, Liu W, Deng J, Zheng N, Gao K, Huang Q, Liu J (2014) Effects of advanced glycation end products on calcium handling in cardiomyocytes. Cardiology 129:75–83. https://doi.org/10.1159/000364779
Petrova R, Yamamoto Y, Muraki K, Yonekura H, Sakurai S, Watanabe T, Li H, Takeuchi M, Makita Z, Kato I, Takasawa S, Okamoto H, Imaizumi Y, Yamamoto H (2002) Advanced glycation endproduct-induced calcium handling impairment in mouse cardiac myocytes. J Mol Cell Cardiol 34:1425–1431
Deng J, Wang G, Huang Q, Yan Y, Li K, Tan W, Jin C, Wang Y, Liu J (2008) Oxidative stress-induced leaky sarcoplasmic reticulum underlying acute heart failure in severe burn trauma. Free Radic Biol Med 44:375–385. https://doi.org/10.1016/j.freeradbiomed.2007.09.023
Ng AC, Delgado V, Bertini M, van der Meer RW, Rijzewijk LJ, Hooi Ewe S, Siebelink HM, Smit JW, Diamant M, Romijn JA, de Roos A, Leung DY, Lamb HJ, Bax JJ (2010) Myocardial steatosis and biventricular strain and strain rate imaging in patients with type 2 diabetes mellitus. Circulation 122:2538–2544. https://doi.org/10.1161/CIRCULATIONAHA.110.955542
Tuncay E, Okatan EN, Toy A, Turan B (2014) Enhancement of cellular antioxidant-defence preserves diastolic dysfunction via regulation of both diastolic Zn2+ and Ca2+ and prevention of RyR2-leak in hyperglycemic cardiomyocytes. Oxidative Med Cell Longev 2014:290381. https://doi.org/10.1155/2014/290381
Gaber EM, Jayaprakash P, Qureshi MA, Parekh K, Oz M, Adrian TE, Howarth FC (2014) Effects of a sucrose-enriched diet on the pattern of gene expression, contraction and Ca(2+) transport in Goto-Kakizaki type 2 diabetic rat heart. Exp Physiol 99:881–893. https://doi.org/10.1113/expphysiol.2013.077594
Sommese L, Valverde CA, Blanco P, Castro MC, Rueda OV, Kaetzel M, Dedman J, Anderson ME, Mattiazzi A, Palomeque J (2016) Ryanodine receptor phosphorylation by CaMKII promotes spontaneous Ca(2+) release events in a rodent model of early stage diabetes: The arrhythmogenic substrate. Int J Cardiol 202:394–406. https://doi.org/10.1016/j.ijcard.2015.09.022
Zhao SM, Wang YL, Guo CY, Chen JL, Wu YQ (2014) Progressive decay of Ca2+ homeostasis in the development of diabetic cardiomyopathy. Cardiovasc Diabetol 13:75. https://doi.org/10.1186/1475-2840-13-75
Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH (2003) Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J Biol Chem 278:44230–44237. https://doi.org/10.1074/jbc.M303810200
Yang X, Su K, Roos MD, Chang Q, Paterson AJ, Kudlow JE (2001) O-linkage of N-acetylglucosamine to Sp1 activation domain inhibits its transcriptional capability. Proc Natl Acad Sci U S A 98:6611–6616. https://doi.org/10.1073/pnas.111099998
Baker DL, Dave V, Reed T, Periasamy M (1996) Multiple Sp1 binding sites in the cardiac/slow twitch muscle sarcoplasmic reticulum Ca2+-ATPase gene promoter are required for expression in Sol8 muscle cells. J Biol Chem 271:5921–5928
Santulli G, Pagano G, Sardu C, Xie W, Reiken S, D'Ascia SL, Cannone M, Marziliano N, Trimarco B, Guise TA, Lacampagne A, Marks AR (2015) Calcium release channel RyR2 regulates insulin release and glucose homeostasis. J Clin Invest 125:1968–1978. https://doi.org/10.1172/JCI79273
Contreras-Ferrat A, Lavandero S, Jaimovich E, Klip A (2014) Calcium signaling in insulin action on striated muscle. Cell Calcium 56:390–396. https://doi.org/10.1016/j.ceca.2014.08.012
Tuncay E, Bitirim CV, Olgar Y, Durak A, Rutter GA, Turan B (2018) Zn(2+)-transporters ZIP7 and ZnT7 play important role in progression of cardiac dysfunction via affecting sarco(endo)plasmic reticulum-mitochondria coupling in hyperglycemic cardiomyocytes. Mitochondrion. https://doi.org/10.1016/j.mito.2017.12.011
Tuncay E, Turan B (2016) Intracellular Zn(2+) increase in cardiomyocytes induces both electrical and mechanical dysfunction in heart via endogenous generation of reactive nitrogen species. Biol Trace Elem Res 169:294–302. https://doi.org/10.1007/s12011-015-0423-3
Tuncay E, Okatan EN, Vassort G, Turan B (2013) Ss-Blocker timolol prevents arrhythmogenic Ca(2)(+) release and normalizes Ca(2)(+) and Zn(2)(+) dyshomeostasis in hyperglycemic rat heart. PloS One 8:e71014. https://doi.org/10.1371/journal.pone.0071014
Olgar Y, Durak A, Tuncay E, Bitirim CV, Ozcinar E, Inan MB, Tokcaer-Keskin Z, Akcali KC, Akar AR, Turan B (2018) Increased free Zn(2+) correlates induction of sarco(endo)plasmic reticulum stress via altered expression levels of Zn(2+)-transporters in heart failure. J Cell Mol Med. https://doi.org/10.1111/jcmm.13480
Olgar Y, Ozdemir S, Turan B (2017) Induction of endoplasmic reticulum stress and changes in expression levels of Zn(2+)-transporters in hypertrophic rat heart. Mol Cell Biochem. https://doi.org/10.1007/s11010-017-3168-9
Woodier J, Rainbow RD, Stewart AJ, Pitt SJ (2015) Intracellular zinc modulates cardiac ryanodine receptor-mediated calcium release. J Biol Chem 290:17599–17610. https://doi.org/10.1074/jbc.M115.661280
Reilly-O'Donnell B, Robertson GB, Karumbi A, McIntyre C, Bal W, Nishi M, Takeshima H, Stewart AJ, Pitt SJ (2017) Dysregulated Zn(2+) homeostasis impairs cardiac type-2 ryanodine receptor and mitsugumin 23 functions, leading to sarcoplasmic reticulum Ca(2+) leakage. J Biol Chem 292:13361–13373. https://doi.org/10.1074/jbc.M117.781708
Yi T, Cheema Y, Tremble SM, Bell SP, Chen Z, Subramanian M, LeWinter MM, VanBuren P, Palmer BM (2012) Zinc-induced cardiomyocyte relaxation in a rat model of hyperglycemia is independent of myosin isoform. Cardiovasc Diabetol 11:135. https://doi.org/10.1186/1475-2840-11-135
Bai T, Wang F, Mellen N, Zheng Y, Cai L (2016) Diabetic cardiomyopathy: role of the E3 ubiquitin ligase. Am J Phys Endocrinol Metab 310:E473–E483. https://doi.org/10.1152/ajpendo.00467.2015
Portbury AL, Ronnebaum SM, Zungu M, Patterson C, Willis MS (2012) Back to your heart: ubiquitin proteasome system-regulated signal transduction. J Mol Cell Cardiol 52:526–537. https://doi.org/10.1016/j.yjmcc.2011.10.023
Le NT, Takei Y, Shishido T, Woo CH, Chang E, Heo KS, Lee H, Lu Y, Morrell C, Oikawa M, McClain C, Wang X, Tournier C, Molina CA, Taunton J, Yan C, Fujiwara K, Patterson C, Yang J, Abe J (2012) p90RSK targets the ERK5-CHIP ubiquitin E3 ligase activity in diabetic hearts and promotes cardiac apoptosis and dysfunction. Circ Res 110:536–550. https://doi.org/10.1161/CIRCRESAHA.111.254730
Li J, Ma W, Yue G, Tang Y, Kim IM, Weintraub NL, Wang X, Su H (2017) Cardiac proteasome functional insufficiency plays a pathogenic role in diabetic cardiomyopathy. J Mol Cell Cardiol 102:53–60. https://doi.org/10.1016/j.yjmcc.2016.11.013
Tang M, Li J, Huang W, Su H, Liang Q, Tian Z, Horak KM, Molkentin JD, Wang X (2010) Proteasome functional insufficiency activates the calcineurin-NFAT pathway in cardiomyocytes and promotes maladaptive remodelling of stressed mouse hearts. Cardiovasc Res 88:424–433. https://doi.org/10.1093/cvr/cvq217
Hu J, Klein JD, Du J, Wang XH (2008) Cardiac muscle protein catabolism in diabetes mellitus: activation of the ubiquitin-proteasome system by insulin deficiency. Endocrinology 149:5384–5390. https://doi.org/10.1210/en.2008-0132
Adams B, Mapanga RF, Essop MF (2015) Partial inhibition of the ubiquitin-proteasome system ameliorates cardiac dysfunction following ischemia-reperfusion in the presence of high glucose. Cardiovasc Diabetol 14:94. https://doi.org/10.1186/s12933-015-0258-4
Madonna R, Geng YJ, Bolli R, Rokosh G, Ferdinandy P, Patterson C, De Caterina R (2014) Co-activation of nuclear factor-kappaB and myocardin/serum response factor conveys the hypertrophy signal of high insulin levels in cardiac myoblasts. J Biol Chem 289:19585–19598. https://doi.org/10.1074/jbc.M113.540559
Bhuiyan MS, Pattison JS, Osinska H, James J, Gulick J, McLendon PM, Hill JA, Sadoshima J, Robbins J (2013) Enhanced autophagy ameliorates cardiac proteinopathy. J Clin Invest 123:5284–5297. https://doi.org/10.1172/JCI70877
Guo R, Zhang Y, Turdi S, Ren J (2013) Adiponectin knockout accentuates high fat diet-induced obesity and cardiac dysfunction: role of autophagy. Biochim Biophys Acta 1832:1136–1148. https://doi.org/10.1016/j.bbadis.2013.03.013
Amato L, Paolisso G, Cacciatore F, Ferrara N, Ferrara P, Canonico S, Varricchio M, Rengo F (1997) Congestive heart failure predicts the development of non-insulin-dependent diabetes mellitus in the elderly. The Osservatorio Geriatrico Regione Campania Group. Diabetes Metab 23:213–218
Andersson C, Norgaard ML, Hansen PR, Fosbol EL, Schmiegelow M, Weeke P, Olesen JB, Raunso J, Jorgensen CH, Vaag A, Kober L, Torp-Pedersen C, Gislason GH (2010) Heart failure severity, as determined by loop diuretic dosages, predicts the risk of developing diabetes after myocardial infarction: a nationwide cohort study. Eur J Heart Fail 12:1333–1338. https://doi.org/10.1093/eurjhf/hfq160
Mohamedali B, Yost G, Bhat G (2014) Mechanical circulatory support improves diabetic control in patients with advanced heart failure. Eur J Heart Fail 16:1120–1124. https://doi.org/10.1002/ejhf.166
Heck PM, Dutka DP (2009) Insulin resistance and heart failure. Curr Heart Fail Rep 6:89–94
McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Guidelines ESCCfP (2012) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 33:1787–1847. https://doi.org/10.1093/eurheartj/ehs104
Elliott WJ, Meyer PM (2007) Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet 369:201–207. https://doi.org/10.1016/S0140-6736(07)60108-1
Opie LH, Schall R (2004) Old antihypertensives and new diabetes. J Hypertens 22:1453–1458
Bakris GL, Fonseca V, Katholi RE, McGill JB, Messerli FH, Phillips RA, Raskin P, Wright JT Jr, Oakes R, Lukas MA, Anderson KM, Bell DS, Investigators G (2004) Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA 292:2227–2236. https://doi.org/10.1001/jama.292.18.2227
Grygiel-Gorniak B (2014) Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications—a review. Nutr J 13:17. https://doi.org/10.1186/1475-2891-13-17
Rogue A, Spire C, Brun M, Claude N, Guillouzo A (2010) Gene expression changes induced by PPAR gamma agonists in animal and human liver. PPAR Res 2010:325183. https://doi.org/10.1155/2010/325183
Willson TM, Brown PJ, Sternbach DD, Henke BR (2000) The PPARs: from orphan receptors to drug discovery. J Med Chem 43:527–550
Berger J, Moller DE (2002) The mechanisms of action of PPARs. Annu Rev Med 53:409–435. https://doi.org/10.1146/annurev.med.53.082901.104018
Sethi S, Ziouzenkova O, Ni H, Wagner DD, Plutzky J, Mayadas TN (2002) Oxidized omega-3 fatty acids in fish oil inhibit leukocyte-endothelial interactions through activation of PPAR alpha. Blood 100:1340–1346. https://doi.org/10.1182/blood-2002-01-0316
Volker D, Fitzgerald P, Major G, Garg M (2000) Efficacy of fish oil concentrate in the treatment of rheumatoid arthritis. J Rheumatol 27:2343–2346
Norell SE, Ahlbom A, Feychting M, Pedersen NL (1986) Fish consumption and mortality from coronary heart disease. Br Med J 293:426
Chrysohoou C, Metallinos G, Georgiopoulos G, Mendrinos D, Papanikolaou A, Magkas N, Pitsavos C, Vyssoulis G, Stefanadis C, Tousoulis D (2016) Short term omega-3 polyunsaturated fatty acid supplementation induces favorable changes in right ventricle function and diastolic filling pressure in patients with chronic heart failure; a randomized clinical trial. Vasc Pharmacol 79:43–50. https://doi.org/10.1016/j.vph.2016.01.005
Heydari B, Abdullah S, Pottala JV, Shah R, Abbasi S, Mandry D, Francis SA, Lumish H, Ghoshhajra BB, Hoffmann U, Appelbaum E, Feng JH, Blankstein R, Steigner M, McConnell JP, Harris W, Antman EM, Jerosch-Herold M, Kwong RY (2016) Effect of omega-3 acid ethyl esters on left ventricular remodeling after acute myocardial infarction: the OMEGA-REMODEL Randomized Clinical Trial. Circulation 134:378–391. https://doi.org/10.1161/CIRCULATIONAHA.115.019949
Tousoulis D, Plastiras A, Siasos G, Oikonomou E, Verveniotis A, Kokkou E, Maniatis K, Gouliopoulos N, Miliou A, Paraskevopoulos T, Stefanadis C (2014) Omega-3 PUFAs improved endothelial function and arterial stiffness with a parallel antiinflammatory effect in adults with metabolic syndrome. Atherosclerosis 232:10–16. https://doi.org/10.1016/j.atherosclerosis.2013.10.014
Nodari S, Triggiani M, Campia U, Manerba A, Milesi G, Cesana BM, Gheorghiade M, Dei Cas L (2011) Effects of n-3 polyunsaturated fatty acids on left ventricular function and functional capacity in patients with dilated cardiomyopathy. J Am Coll Cardiol 57:870–879. https://doi.org/10.1016/j.jacc.2010.11.017
Berecki G, Den Ruijter HM, Verkerk AO, Schumacher CA, Baartscheer A, Bakker D, Boukens BJ, van Ginneken AC, Fiolet JW, Opthof T, Coronel R (2007) Dietary fish oil reduces the incidence of triggered arrhythmias in pig ventricular myocytes. Heart Rhythm 4:1452–1460. https://doi.org/10.1016/j.hrthm.2007.07.015
Correction (2015) Circulation 131:e535. https://doi.org/10.1161/CIR.0000000000000219
Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, Drury P, Kesaniemi YA, Sullivan D, Hunt D, Colman P, d'Emden M, Whiting M, Ehnholm C, Laakso M, investigators Fs (2005) Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 366:1849–1861. https://doi.org/10.1016/S0140-6736(05)67667-2
(2001) Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomised study. Lancet 357:905–910
Ichihara S, Obata K, Yamada Y, Nagata K, Noda A, Ichihara G, Yamada A, Kato T, Izawa H, Murohara T, Yokota M (2006) Attenuation of cardiac dysfunction by a PPAR-alpha agonist is associated with down-regulation of redox-regulated transcription factors. J Mol Cell Cardiol 41:318–329. https://doi.org/10.1016/j.yjmcc.2006.05.013
Labinskyy V, Bellomo M, Chandler MP, Young ME, Lionetti V, Qanud K, Bigazzi F, Sampietro T, Stanley WC, Recchia FA (2007) Chronic activation of peroxisome proliferator-activated receptor-alpha with fenofibrate prevents alterations in cardiac metabolic phenotype without changing the onset of decompensation in pacing-induced heart failure. J Pharmacol Exp Ther 321:165–171. https://doi.org/10.1124/jpet.106.116871
De Silva DS, Wilson RM, Hutchinson C, Ip PC, Garcia AG, Lancel S, Ito M, Pimentel DR, Sam F (2009) Fenofibrate inhibits aldosterone-induced apoptosis in adult rat ventricular myocytes via stress-activated kinase-dependent mechanisms. Am J Physiol Heart Circ Physiol 296:H1983–H1993. https://doi.org/10.1152/ajpheart.00002.2009
Ogata T, Miyauchi T, Sakai S, Irukayama-Tomobe Y, Goto K, Yamaguchi I (2002) Stimulation of peroxisome-proliferator-activated receptor alpha (PPAR alpha) attenuates cardiac fibrosis and endothelin-1 production in pressure-overloaded rat hearts. Clin Sci 103(Suppl 48):284S–288S. https://doi.org/10.1042/CS103S284S
Liu GZ, Hou TT, Yuan Y, Hang PZ, Zhao JJ, Sun L, Zhao GQ, Zhao J, Dong JM, Wang XB, Shi H, Liu YW, Zhou JH, Dong ZX, Liu Y, Zhan CC, Li Y, Li WM (2016) Fenofibrate inhibits atrial metabolic remodelling in atrial fibrillation through PPAR-alpha/sirtuin 1/PGC-1alpha pathway. Br J Pharmacol 173:1095–1109. https://doi.org/10.1111/bph.13438
Li P, Luo S, Pan C, Cheng X (2015) Modulation of fatty acid metabolism is involved in the alleviation of isoproterenol-induced rat heart failure by fenofibrate. Mol Med Rep 12:7899–7906. https://doi.org/10.3892/mmr.2015.4466
Hafstad AD, Khalid AM, Hagve M, Lund T, Larsen TS, Severson DL, Clarke K, Berge RK, Aasum E (2009) Cardiac peroxisome proliferator-activated receptor-alpha activation causes increased fatty acid oxidation, reducing efficiency and post-ischaemic functional loss. Cardiovasc Res 83:519–526. https://doi.org/10.1093/cvr/cvp132
Huang WP, Yin WH, Chen JW, Jen HL, Young MS, Lin SJ (2009) Fenofibrate attenuates endothelial monocyte adhesion in chronic heart failure: an in vitro study. Eur J Clin Investig 39:775–783. https://doi.org/10.1111/j.1365-2362.2009.02176.x
Lebrasseur NK, Duhaney TA, De Silva DS, Cui L, Ip PC, Joseph L, Sam F (2007) Effects of fenofibrate on cardiac remodeling in aldosterone-induced hypertension. Hypertension 50:489–496. https://doi.org/10.1161/hypertensionaha.107.092403
Duhaney TA, Cui L, Rude MK, Lebrasseur NK, Ngoy S, De Silva DS, Siwik DA, Liao R, Sam F (2007) Peroxisome proliferator-activated receptor alpha-independent actions of fenofibrate exacerbates left ventricular dilation and fibrosis in chronic pressure overload. Hypertension 49:1084–1094. https://doi.org/10.1161/hypertensionaha.107.086926
Brigadeau F, Gele P, Wibaux M, Marquie C, Martin-Nizard F, Torpier G, Fruchart JC, Staels B, Duriez P, Lacroix D (2007) The PPARalpha activator fenofibrate slows down the progression of the left ventricular dysfunction in porcine tachycardia-induced cardiomyopathy. J Cardiovasc Pharmacol 49:408–415. https://doi.org/10.1097/FJC.0b013e3180544540
Morgan EE, Rennison JH, Young ME, McElfresh TA, Kung TA, Tserng KY, Hoit BD, Stanley WC, Chandler MP (2006) Effects of chronic activation of peroxisome proliferator-activated receptor-alpha or high-fat feeding in a rat infarct model of heart failure. Am J Phys Heart Circ Phys 290:H1899–H1904. https://doi.org/10.1152/ajpheart.01014.2005
Yeh CH, Chen TP, Lee CH, Wu YC, Lin YM, Lin PJ (2006) Cardiomyocytic apoptosis following global cardiac ischemia and reperfusion can be attenuated by peroxisome proliferator-activated receptor alpha but not gamma activators. Shock 26:262–270. https://doi.org/10.1097/01.shk.0000225863.56714.96
Dewald O, Sharma S, Adrogue J, Salazar R, Duerr GD, Crapo JD, Entman ML, Taegtmeyer H (2005) Downregulation of peroxisome proliferator-activated receptor-alpha gene expression in a mouse model of ischemic cardiomyopathy is dependent on reactive oxygen species and prevents lipotoxicity. Circulation 112:407–415. https://doi.org/10.1161/circulationaha.105.536318
Yue TL, Bao W, Jucker BM, Gu JL, Romanic AM, Brown PJ, Cui J, Thudium DT, Boyce R, Burns-Kurtis CL, Mirabile RC, Aravindhan K, Ohlstein EH (2003) Activation of peroxisome proliferator-activated receptor-alpha protects the heart from ischemia/reperfusion injury. Circulation 108:2393–2399. https://doi.org/10.1161/01.cir.0000093187.42015.6c
Young ME, Laws FA, Goodwin GW, Taegtmeyer H (2001) Reactivation of peroxisome proliferator-activated receptor alpha is associated with contractile dysfunction in hypertrophied rat heart. J Biol Chem 276:44390–44395. https://doi.org/10.1074/jbc.M103826200
Yu BC, Chang CK, Ou HY, Cheng KC, Cheng JT (2008) Decrease of peroxisome proliferator-activated receptor delta expression in cardiomyopathy of streptozotocin-induced diabetic rats. Cardiovasc Res 80:78–87. https://doi.org/10.1093/cvr/cvn172
Henke BR (2004) Peroxisome proliferator-activated receptor alpha/gamma dual agonists for the treatment of type 2 diabetes. J Med Chem 47:4118–4127. https://doi.org/10.1021/jm030631e
Madrid-Miller A, Moreno-Ruiz LA, Borrayo-Sanchez G, Almeida-Gutierrez E, Martinez-Gomez DF, Jauregui-Aguilar R (2010) Impact of bezafibrate treatment in patients with hyperfibrinogenemia and ST-elevation acute myocardial infarction: a randomized clinical trial. Cir Cir 78:229–237
Feige JN, Gelman L, Michalik L, Desvergne B, Wahli W (2006) From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog Lipid Res 45:120–159. https://doi.org/10.1016/j.plipres.2005.12.002
Medina-Gomez G, Gray SL, Yetukuri L, Shimomura K, Virtue S, Campbell M, Curtis RK, Jimenez-Linan M, Blount M, Yeo GS, Lopez M, Seppanen-Laakso T, Ashcroft FM, Oresic M, Vidal-Puig A (2007) PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet 3:e64. https://doi.org/10.1371/journal.pgen.0030064
Sarma S (2012) Use of clinically available PPAR agonists for heart failure; do the risks outweigh the potential benefits? Curr Mol Pharmacol 5:255–263
Qu A, Shah YM, Manna SK, Gonzalez FJ (2012) Disruption of endothelial peroxisome proliferator-activated receptor gamma accelerates diet-induced atherogenesis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol 32:65–73. https://doi.org/10.1161/ATVBAHA.111.239137
Pelham CJ, Keen HL, Lentz SR, Sigmund CD (2013) Dominant negative PPARgamma promotes atherosclerosis, vascular dysfunction, and hypertension through distinct effects in endothelium and vascular muscle. Am J Physiol Regul Integr Comp Physiol 304:R690–R701. https://doi.org/10.1152/ajpregu.00607.2012
Lehrke M, Marx N (2017) Diabetes mellitus and heart failure. Am J Med 130:S40–S50. https://doi.org/10.1016/j.amjmed.2017.04.010
Tousoulis D, Psaltopoulou T, Androulakis E, Papageorgiou N, Papaioannou S, Oikonomou E, Synetos A, Stefanadis C (2015) Oxidative stress and early atherosclerosis: novel antioxidant treatment. Cardiovasc Drugs Ther sponsored by the International Society of Cardiovascular Pharmacotherapy 29:75–88. https://doi.org/10.1007/s10557-014-6562-5
Sarafidis PA, Lasaridis AN, Nilsson PM, Mouslech TF, Hitoglou-Makedou AD, Stafylas PC, Kazakos KA, Yovos JG, Tourkantonis AA (2005) The effect of rosiglitazone on novel atherosclerotic risk factors in patients with type 2 diabetes mellitus and hypertension. An open-label observational study. Metabolism 54:1236–1242. https://doi.org/10.1016/j.metabol.2005.04.010
Lebovitz HE, Banerji MA (2001) Insulin resistance and its treatment by thiazolidinediones. Recent Prog Horm Res 56:265–294
Krishnaswami A, Ravi-Kumar S, Lewis JM (2010) Thiazolidinediones: a 2010 perspective. Perm J 14:64–72
Miyazaki Y, Mahankali A, Matsuda M, Mahankali S, Hardies J, Cusi K, Mandarino LJ, DeFronzo RA (2002) Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 87:2784–2791. https://doi.org/10.1210/jcem.87.6.8567
Derosa G, Dangelo A, Ragonesi PD, Ciccarelli L, Piccinni MN, Pricolo F, Salvadeo S, Montagna L, Gravina A, Ferrari I, Galli S, Paniga S, Cicero AF (2006) Effects of rosiglitazone and pioglitazone combined with metformin on the prothrombotic state of patients with type 2 diabetes mellitus and metabolic syndrome. J Int Med Res 34:545–555. https://doi.org/10.1177/147323000603400513
Kruszynska YT, Yu JG, Olefsky JM, Sobel BE (2000) Effects of troglitazone on blood concentrations of plasminogen activator inhibitor 1 in patients with type 2 diabetes and in lean and obese normal subjects. Diabetes 49:633–639
Ji Y, Liu J, Wang Z, Liu N, Gou W (2009) PPARgamma agonist, rosiglitazone, regulates angiotensin II-induced vascular inflammation through the TLR4-dependent signaling pathway. Lab Invest 89:887–902. https://doi.org/10.1038/labinvest.2009.45
Diep QN, Amiri F, Benkirane K, Paradis P, Schiffrin EL (2004) Long-term effects of the PPAR gamma activator pioglitazone on cardiac inflammation in stroke-prone spontaneously hypertensive rats. Can J Physiol Pharmacol 82:976–985. https://doi.org/10.1139/y04-094
Patel CB, De Lemos JA, Wyne KL, McGuire DK (2006) Thiazolidinediones and risk for atherosclerosis: pleiotropic effects of PPar gamma agonism. Diab Vasc Dis Res 3:65–71. https://doi.org/10.3132/dvdr.2006.016
Bagi Z, Koller A, Kaley G (2004) PPARgamma activation, by reducing oxidative stress, increases NO bioavailability in coronary arterioles of mice with type 2 diabetes. Am J Phys Heart Circ Phys 286:H742–H748. https://doi.org/10.1152/ajpheart.00718.2003
Nakamura T, Matsuda T, Kawagoe Y, Ogawa H, Takahashi Y, Sekizuka K, Koide H (2004) Effect of pioglitazone on carotid intima-media thickness and arterial stiffness in type 2 diabetic nephropathy patients. Metab Clin Exp 53:1382–1386
Qayyum R, Adomaityte J (2006) A meta-analysis of the effect of thiazolidinediones on blood pressure. J Clin Hypertens 8:19–28
Giri SR, Bhoi B, Jain MR, Gatne MM (2016) Cardioprotective role of peroxisome proliferator-activated receptor-gamma agonist, rosiglitazone in a unique murine model of diabetic cardiopathy. Life Sci 162:1–13. https://doi.org/10.1016/j.lfs.2016.08.013
Kannan S, Pantalone KM, Matsuda S, Wells BJ, Karafa M, Zimmerman RS (2016) Risk of overall mortality and cardiovascular events in patients with type 2 diabetes on dual drug therapy including metformin: a large database study from the Cleveland Clinic. J Diabetes 8:279–285. https://doi.org/10.1111/1753-0407.12301
Choy-Shan A, Zinn A, Shah B, Danoff A, Donnino R, Schwartzbard AZ, Lorin JD, Grossi E, Sedlis SP (2012) Effect of rosiglitazone on survival in patients with diabetes mellitus treated for coronary artery disease. Coron Artery Dis 23:354–358. https://doi.org/10.1097/MCA.0b013e3283564897
Eurich DT, McAlister FA, Blackburn DF, Majumdar SR, Tsuyuki RT, Varney J, Johnson JA (2007) Benefits and harms of antidiabetic agents in patients with diabetes and heart failure: systematic review. BMJ 335:497. https://doi.org/10.1136/bmj.39314.620174.80
Dormandy J, Bhattacharya M, van Troostenburg de Bruyn AR, investigators PR (2009) Safety and tolerability of pioglitazone in high-risk patients with type 2 diabetes: an overview of data from PROactive. Drug Saf 32:187–202. https://doi.org/10.2165/00002018-200932030-00002
Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Koranyi L, Laakso M, Mokan M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J, Investigators PR (2005) Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366:1279–1289. https://doi.org/10.1016/S0140-6736(05)67528-9
Yue TL (2003) Cardioprotective effects of thiazolidinediones, peroxisome proliferator-activated receptor-gamma agonists. Drugs Today 39:949–960
Yue Tl TL, Chen J, Bao W, Narayanan PK, Bril A, Jiang W, Lysko PG, Gu JL, Boyce R, Zimmerman DM, Hart TK, Buckingham RE, Ohlstein EH (2001) In vivo myocardial protection from ischemia/reperfusion injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation 104:2588–2594
Huang JV, Greyson CR, Schwartz GG (2012) PPAR-gamma as a therapeutic target in cardiovascular disease: evidence and uncertainty. J Lipid Res 53:1738–1754. https://doi.org/10.1194/jlr.R024505
Sarraf M, Lu L, Ye S, Reiter MJ, Greyson CR, Schwartz GG (2012) Thiazolidinedione drugs promote onset, alter characteristics, and increase mortality of ischemic ventricular fibrillation in pigs. Cardiovasc Drugs Ther 26:195–204. https://doi.org/10.1007/s10557-012-6384-2
Chintalgattu V, Harris GS, Akula SM, Katwa LC (2007) PPAR-gamma agonists induce the expression of VEGF and its receptors in cultured cardiac myofibroblasts. Cardiovasc Res 74:140–150. https://doi.org/10.1016/j.cardiores.2007.01.010
Shiomi T, Tsutsui H, Hayashidani S, Suematsu N, Ikeuchi M, Wen J, Ishibashi M, Kubota T, Egashira K, Takeshita A (2002) Pioglitazone, a peroxisome proliferator-activated receptor-gamma agonist, attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation 106:3126–3132
Nemoto S, Razeghi P, Ishiyama M, De Freitas G, Taegtmeyer H, Carabello BA (2005) PPAR-gamma agonist rosiglitazone ameliorates ventricular dysfunction in experimental chronic mitral regurgitation. Am J Phys Heart Circ Phys 288:H77–H82. https://doi.org/10.1152/ajpheart.01246.2003
Lkhagva B, Lin YK, Kao YH, Chazo TF, Chung CC, Chen SA, Chen YJ (2015) Novel histone deacetylase inhibitor modulates cardiac peroxisome proliferator-activated receptors and inflammatory cytokines in heart failure. Pharmacology 96:184–191. https://doi.org/10.1159/000438864
Dargie HJ, Hildebrandt PR, Riegger GA, McMurray JJ, McMorn SO, Roberts JN, Zambanini A, Wilding JP (2007) A randomized, placebo-controlled trial assessing the effects of rosiglitazone on echocardiographic function and cardiac status in type 2 diabetic patients with New York Heart Association Functional Class I or II Heart Failure. J Am Coll Cardiol 49:1696–1704. https://doi.org/10.1016/j.jacc.2006.10.077
Yamamoto K, Ohki R, Lee RT, Ikeda U, Shimada K (2001) Peroxisome proliferator-activated receptor gamma activators inhibit cardiac hypertrophy in cardiac myocytes. Circulation 104:1670–1675
Sakamoto A, Hongo M, Furuta K, Saito K, Nagai R, Ishizaka N (2013) Pioglitazone ameliorates systolic and diastolic cardiac dysfunction in rat model of angiotensin II-induced hypertension. Int J Cardiol 167:409–415. https://doi.org/10.1016/j.ijcard.2012.01.007
Kim SK, Zhao ZS, Lee YJ, Lee KE, Kang SM, Choi D, Lim SK, Chung N, Lee HC, Cha BS (2003) Left-ventricular diastolic dysfunction may be prevented by chronic treatment with PPAR-alpha or -gamma agonists in a type 2 diabetic animal model. Diabetes Metab Res Rev 19:487–493. https://doi.org/10.1002/dmrr.410
Rodriguez WE, Joshua IG, Falcone JC, Tyagi SC (2006) Pioglitazone prevents cardiac remodeling in high-fat, high-calorie-induced type 2 diabetes mellitus. Am J Phys Heart Circ Phys 291:H81–H87. https://doi.org/10.1152/ajpheart.01331.2005
Zhao SM, Li HW, Guo CY, Shen LH (2010) Cardiac fibrosis in diabetic rats: regulation and mechanism of activation of the PPARgamma signal pathway. Chin J Physiol 53:262–267
Duan SZ, Ivashchenko CY, Russell MW, Milstone DS, Mortensen RM (2005) Cardiomyocyte-specific knockout and agonist of peroxisome proliferator-activated receptor-gamma both induce cardiac hypertrophy in mice. Circ Res 97:372–379. https://doi.org/10.1161/01.RES.0000179226.34112.6d
Horio T, Suzuki M, Suzuki K, Takamisawa I, Hiuge A, Kamide K, Takiuchi S, Iwashima Y, Kihara S, Funahashi T, Yoshimasa Y, Kawano Y (2005) Pioglitazone improves left ventricular diastolic function in patients with essential hypertension. Am J Hypertens 18:949–957. https://doi.org/10.1016/j.amjhyper.2005.02.003
Ordu S, Ozhan H, Alemdar R, Aydin M, Basar C, Caglar O, Yazici M, Yalcin S (2010) Pioglitazone improves ventricular diastolic function in patients with diabetes mellitus: a tissue Doppler study. Acta Cardiol 65:401–406. https://doi.org/10.2143/ac.65.4.2053898
Naka KK, Pappas K, Papathanassiou K, Papamichael ND, Kazakos N, Kanioglou C, Makriyiannis D, Katsouras CS, Liveris K, Tsatsoulis A, Michalis LK (2010) Lack of effects of pioglitazone on cardiac function in patients with type 2 diabetes and evidence of left ventricular diastolic dysfunction: a tissue Doppler imaging study. Cardiovasc Diabetol 9:57. https://doi.org/10.1186/1475-2840-9-57
Sakai S, Miyauchi T, Irukayama-Tomobe Y, Ogata T, Goto K, Yamaguchi I (2002) Peroxisome proliferator-activated receptor-gamma activators inhibit endothelin-1-related cardiac hypertrophy in rats. Clin Sci 103(Suppl 48):16S–20S. https://doi.org/10.1042/CS103S016S
Meng Z, Yu XH, Chen J, Li L, Li S (2014) Curcumin attenuates cardiac fibrosis in spontaneously hypertensive rats through PPAR-gamma activation. Acta Pharmacol Sin 35:1247–1256. https://doi.org/10.1038/aps.2014.63
Wu J, Hu W, Gong Y, Wang P, Tong L, Chen X, Chen Z, Xu X, Yao W, Zhang W, Huang C (2017) Current pharmacological developments in 2,3,4′,5-tetrahydroxystilbene 2-O-beta-D-glucoside (TSG). Eur J Pharmacol. https://doi.org/10.1016/j.ejphar.2017.05.037
Peng Y, Zeng Y, Xu J, Huang XL, Zhang W, Xu XL (2016) PPAR-gamma is involved in the protective effect of 2,3,4′,5-tetrahydroxystilbene-2-O-beta-D-glucoside against cardiac fibrosis in pressure-overloaded rats. Eur J Pharmacol 791:105–114. https://doi.org/10.1016/j.ejphar.2016.08.025
Hao GH, Niu XL, Gao DF, Wei J, Wang NP (2008) Agonists at PPAR-gamma suppress angiotensin II-induced production of plasminogen activator inhibitor-1 and extracellular matrix in rat cardiac fibroblasts. Br J Pharmacol 153:1409–1419. https://doi.org/10.1038/bjp.2008.21
Chen X, Bing Z, He J, Jiang L, Luo X, Su Y, Kan B, Huang D, Wei Y (2009) Downregulation of peroxisome proliferator-activated receptor-gamma expression in hypertensive atrial fibrillation. Clin Cardiol 32:337–345. https://doi.org/10.1002/clc.20566
Wang Y, Huang S, Sah VP, Ross J Jr, Brown JH, Han J, Chien KR (1998) Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem 273:2161–2168
Swynghedauw B (1999) Molecular mechanisms of myocardial remodeling. Physiol Rev 79:215–262
Chen K, Chen J, Li D, Zhang X, Mehta JL (2004) Angiotensin II regulation of collagen type I expression in cardiac fibroblasts: modulation by PPAR-gamma ligand pioglitazone. Hypertension 44:655–661. https://doi.org/10.1161/01.HYP.0000144400.49062.6b
Liu HJ, Liao HH, Yang Z, Tang QZ (2016) Peroxisome proliferator-activated receptor-gamma is critical to cardiac fibrosis. PPAR Res 2016:2198645. https://doi.org/10.1155/2016/2198645
Claessen BE, Stone GW, Mehran R, Witzenbichler B, Brodie BR, Wohrle J, Witkowski A, Guagliumi G, Zmudka K, Henriques JP, Tijssen JG, Sanidas EA, Chantziara V, Hakim D, Leon S, Xu K, Dangas GD (2012) Relationship between biomarkers and subsequent clinical and angiographic restenosis after paclitaxel-eluting stents for treatment of STEMI: a HORIZONS-AMI substudy. J Thromb Thrombolysis 34:165–179. https://doi.org/10.1007/s11239-012-0706-x
Robinson JG (2008) Should we use PPAR agonists to reduce cardiovascular risk? PPAR Res 2008:891425. https://doi.org/10.1155/2008/891425
Inzucchi SE, Masoudi FA, Wang Y, Kosiborod M, Foody JM, Setaro JF, Havranek EP, Krumholz HM (2005) Insulin-sensitizing antihyperglycemic drugs and mortality after acute myocardial infarction: insights from the National Heart Care Project. Diabetes Care 28:1680–1689
Nissen SE, Wolski K (2007) Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356:2457–2471. https://doi.org/10.1056/NEJMoa072761
Sena S, Rasmussen IR, Wende AR, McQueen AP, Theobald HA, Wilde N, Pereira RO, Litwin SE, Berger JP, Abel ED (2007) Cardiac hypertrophy caused by peroxisome proliferator-activated receptor-gamma agonist treatment occurs independently of changes in myocardial insulin signaling. Endocrinology 148:6047–6053. https://doi.org/10.1210/en.2006-1559
Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Le Winter M, Porte D, Semenkovich CF, Smith S, Young LH, Kahn R (2004) Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care 27:256–263
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
The manuscript does not contain clinical studies or patient data.
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Oikonomou, E., Mourouzis, K., Fountoulakis, P. et al. Interrelationship between diabetes mellitus and heart failure: the role of peroxisome proliferator-activated receptors in left ventricle performance. Heart Fail Rev 23, 389–408 (2018). https://doi.org/10.1007/s10741-018-9682-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-018-9682-3