Abstract
This systematic review and meta-analysis aimed to evaluate the effects of cognitive behavioural therapy (CBT) on depression, quality of life, hospitalisations and mortality in heart failure patients. The search strategy was developed for Ovid MEDLINE and modified accordingly to search the following bibliographic databases: PubMed, EMBASE, PsycINFO, CENTRAL and CINAHL. Databases were searched from inception to 6 March 2016 for randomised controlled trials (RCTs) or observational studies that used CBT in heart failure patients with depression or depressive symptoms. Six studies were identified: 5 RCTs and 1 observational study, comprising 320 participants with predominantly NYHA classes II-III, who were mostly male, with mean age ranging from 55 to 66 years. Compared to usual care, CBT was associated with a greater improvement in depression scores both initially after CBT sessions (standardised mean difference −0.34, 95% CI −0.60 to −0.08, p = 0.01) and at 3 months follow-up (standardised mean difference −0.32, 95% CI −0.59 to −0.04, p = 0.03). Greater improvement in quality of life scores was evident for the CBT group initially after CBT sessions, but with no difference at 3 months. Hospital admissions and mortality were similar, regardless of treatment group. CBT may be more effective than usual care at improving depression scores and quality of life for heart failure patients initially following CBT and for depression at 3 months. Larger and more robust RCTs are needed to evaluate the long-term clinical effects of CBT in heart failure patients.
Similar content being viewed by others
Introduction
Heart failure continues to impose a tremendous burden on patients, carers and healthcare systems. In Europe, approximately 6.5 million people are currently living with the condition [1], with a prevalence of ≥10% among the 70 years and older population [2]. Heart failure is the endpoint of all cardiovascular diseases [3], and therefore, the improved survival rates for other cardiovascular diseases are expected to further increase the prevalence, in addition to the increase due to the ageing population [2]. It is a significant cause of mortality, with approximately 5% of all deaths attributable to heart failure in the UK [4], and only 25% of patients are expected to survive beyond 5 years after their first hospital admission [5]—a prognosis that is worse than most cancers [6]. The morbidity associated with heart failure costs the UK National Health Service (NHS) around 2% of its annual budget, which is primarily through costs associated with hospitalisation [7]. Two percent of all hospitalised bed-days and 5% of all emergency hospital admissions are a result of heart failure [4, 8].
Depression is characterised by symptoms that affect a patient’s cognitive, emotional and behavioural processes [9]. The association between depression and heart failure has been demonstrated in numerous studies; however, the specific rate of prevalence varies, ranging from 9% [10] to as much as 60% [11], with one meta-analysis reporting a pooled estimate of 22% [12]. The existence of depression has negative implications for heart failure patients, particularly through reduced survival and an increased risk of secondary events [12,13,14,15]. The precise mechanism by which depression causes poorer outcomes in heart failure patients is unclear, but is thought to be a combination of behavioural influences and their interaction with physiological responses [16,17,18]. In addition, the behavioural influences of depression can reduce the likelihood of both treatment adherence [19] and modifying lifestyle behaviours [16], which may further contribute to adverse outcomes.
Cognitive behavioural therapy (CBT) refers to a group of psychological interventions that aim to understand a patient’s normal cognitive and behavioural processes, and modify these to eliminate negative cognitions and behaviours [20, 21]. CBT is a well-established intervention in depression, and is currently recommended in guidelines [22], but its effectiveness for depression in heart failure patients remains unclear [23]. Two previous systematic reviews only identified one study that had examined psychological interventions for depression in heart failure patients [3, 24]. For a condition with an already poor prognosis and reduced quality of life, the potential for further deterioration with concomitant depression needs to be addressed. This systematic review evaluated the effectiveness of CBT for heart failure patients with depression by assessing changes in depression scores, impact on quality of life (QoL) and rates of hospitalisation and mortality.
Methods
This systematic review and meta-analysis was prospectively registered with the PROSPERO database of systematic reviews (CRD42016036146) [25] and reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [26].
Study selection
The search strategy was developed by the research team and checked by an information specialist prior to implementation (see Supplementary material 1). The strategy included specific terms relevant to the study objectives: CBT, heart failure and depression. It was primarily developed for Ovid MEDLINE, before adaptation for use in other bibliographic databases. The following bibliographic databases were searched from inception to 6 March 2016: PubMed/MEDLINE, EMBASE, PsycINFO, Cochrane Central Register of Controlled Trials (CENTRAL) and CINAHL. Grey literature was found through contacting lead authors on heart failure for unpublished studies and through manual searches of reference lists of included papers. Two reviewers independently screened the titles, abstracts and full texts of studies, as appropriate, for potentially relevant studies. Disagreements were resolved through discussion or adjudication by a third person.
Inclusion and exclusion criteria
Both randomised controlled trials (RCTs) and observational studies were eligible for inclusion. Studies were only included if they met the following criteria: participants were ≥18 years, with a clinical diagnosis of heart failure as defined by each study (usually relating to a combination of clinical symptoms and identification of either systolic or diastolic dysfunction) and with depression or depressive symptoms (above or equal to a predefined cut-off on validated depression questionnaires). The intervention was CBT, as described by authors, entailing both cognitive and behavioural components; studies comprising solely of cognitive or behavioural therapies were excluded, as were CBT interventions as part of a more comprehensive package (e.g. with exercise). Comparators included usual care, exercise, medication or no treatment. There were no language restrictions imposed, but accessibility of full-text publication was a requirement. Two reviewers independently determined eligibility for inclusion/exclusion, with disagreements discussed and referred to a third reviewer if required.
Outcomes
The following outcomes were of interest: change in depression (assessed through changes to depression scores on validated questionnaires), quality of life (assessed by a validated quality of life questionnaire) and clinical outcomes of hospitalisation and mortality (both all-cause mortality and cardiovascular mortality). However, there was insufficient information on cause of death to examine the impact of CBT on cardiovascular mortality.
Data extraction and risk of bias assessment
Two reviewers used a standardised data extraction form to independently extract the following information: study population (baseline characteristics, sample size, definition of heart failure and assessment of depression/depressive symptoms), CBT intervention (description of CBT, duration and frequency), comparator (type of comparator, duration and frequency) and the outcomes (method of assessment, depression scores, quality of life scores, number of hospitalisations and deaths). Included studies were assessed for methodological quality using risk of bias assessment tools. For RCTs, the Cochrane Risk of Bias tool [27] was used, which considers selection bias (randomisation and allocation concealment), performance bias (blinding of participants and comparators), detection bias (blinding of assessed outcomes), attrition bias (incomplete outcome data) and reporting bias. For the assessment of observational studies, the Risk of Bias Assessment tool for Non-randomised Studies (RoBANS) [28] was employed.
Statistical analysis
The outcome data from included studies were reported as either dichotomous or continuous variables. For dichotomous data, the risk ratio and 95% confidence intervals were reported. For continuous variables, the mean differences (MD) between CBT and comparator groups were reported, with standardised mean differences (SMD) and 95% confidence intervals calculated. In studies where the mean difference was not reported, it was calculated for relevant time-points by using individual patient data obtained from the authors of individual studies. A meta-analysis of quantitative data was performed using fixed-effects modelling, under the assumption that there would be a similar effect from trials with similar patient populations and outcomes [29]. As a sensitivity analysis, we performed random effects modelling using the method of DerSimonian and Laird [30]. For outcomes measured by different questionnaires, subgroup analyses were performed separately for all questionnaires and for questionnaires which were common across included studies. All effect estimates were accompanied by 95% confidence intervals and assessed for heterogeneity (using the I 2 statistic). All statistical tests were two-tailed, and p values of <0.05 were considered statistically significant. Meta-analysis was performed using Review Manager (RevMan) version 5.3 (The Nordic Cochrane Centre, Copenhagen, 2014).
Results
Identification and selection of studies
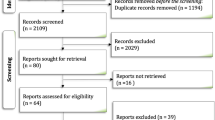
The searches revealed a total of 454 papers, reduced to 298 papers after removing duplicates (Fig. 1). After independent screening of titles and abstracts, 287 papers were determined ineligible, with 11 papers retrieved for further evaluation. An additional three papers were obtained through contacting lead authors and grey literature search, resulting in 14 papers for further evaluation. Of these, four papers were excluded due to inappropriate populations [31,32,33,34]; three papers were excluded as information on heart failure patients could not be isolated from the study population, despite contacting authors [35,36,37]; two papers were excluded due to inappropriate interventions [38, 39] (see Supplementary material 2). Of the remaining five papers [40,41,42,43,44], one was an abstract that combined data for two separate RCTs [44]; therefore, the lead author was contacted, and provided the individual patient data and corresponding protocols for the two studies (referred to as Dekker, 2010 Personal Communication and Dekker, 2011 Personal Communication from here on). Therefore, six studies were included in this systematic review.
Description of included studies
The six studies comprised 320 participants with predominantly New York Heart Association (NYHA) classes II-III, who were mostly male (ranging from 43 to 70% across studies), with mean ages ranging from 55 to 66 years (Table 1). Five were RCTs [40,41,42 Dekker 2010, Dekker 2011], and one study was observational [43]. Four of the RCTs had one intervention group whilst one [42] had three intervention groups (one was a combination of CBT with exercise and was excluded from these analyses). In all the RCTs, the CBT intervention consisted of individual, face-to-face sessions that were delivered by an experienced nurse or therapist. These sessions varied in frequency and duration: three studies had single sessions each lasting 30 min [40, Dekker 2010, Dekker 2011]; one study had weekly sessions lasting 60 min for 12 weeks [42]; and one study initially had 60-min weekly sessions, before tapering to bi-weekly and then bi-monthly sessions, for a total of 6 months [41]. The CBT sessions generally involved building rapport with patients, understanding patient thoughts and behaviours about heart failure, educating patients about depression and setting and reviewing assignments. For all RCTs, the face-to-face CBT sessions were followed up by ‘booster’ CBT telephone sessions. The comparator in the RCTs was usual care only, with one study having a second, exercise only comparator group. The observational study was a proof-of-concept study, which consisted of an Internet-based programme that delivered CBT modules over a 9-week period, with contact with healthcare professionals only through written feedback on assignments. Attrition rates were low across studies, except for two studies [Dekker 2010; Dekker 2011], where there were significant losses to follow-up beyond the 1 week time-point (Table 1).
Risk of bias
For the five RCTs, the process of randomisation was described in adequate detail for three studies [40 Dekker 2010, Dekker 2011], but for two studies, the precise method of random sequence generation was not clear [41, 42]. Allocation concealment was only explained thoroughly in Freedland et al. [41]. Given the nature of the CBT intervention, blinding of participants was not possible; only two studies sufficiently explained the blinding of outcome assessors [40, 41] (Fig. 2). Incomplete outcome data was addressed in three RCTs with appropriate reasons provided for attrition and similar rates between groups [40,41,42]. Selective reporting was difficult to ascertain due to a lack of published protocols. The RCTs were free from other sources of bias, except for one study [40] with potential social desirability bias due to the follow-up questionnaires being administered by the same nurse that delivered the intervention. For the observational study, the nature of the CBT intervention could have resulted in sampling bias, as patients required access to the Internet and basic computer skills. Further, the level of exposure participants had to the intervention is unclear, as no healthcare professionals were present during the intervention itself. Some potential confounding variables were not included (i.e. severity of heart failure), but there were no signs of attrition bias or selective outcome reporting. Small study bias could not be assessed by testing for funnel plot asymmetry as there were <10 studies [29] in this meta-analysis.
Outcomes
For depression and quality of life, the effects of CBT were evaluated at two time-points: the first time-point was after the main CBT phase and again 3 months later, due to the differing lengths of CBT and follow-up points in each trial (Table 1; Fig. 3).
Depression
The change in depressive symptoms was assessed by depression scores on validated questionnaires. All the RCTs used mean Beck’s Depression Inventory (BDI-II) scores, except one [41], which used the Hamilton Rating Scale for Depression (HAM-D); some also used an additional depression questionnaire (HAM-D or Patient Health Questionnaire (PHQ-9) [41, 42 Dekker 2010, Dekker 2011]. The observational study [43] used the PHQ-9 and Montgomery-Åsberg Depression Rating Scale (MADRS) and was not included in the meta-analysis. This study [43] demonstrated a decrease in median depression scores from baseline (PHQ-9 11, MADRS 25.5) to 9 weeks (PHQ-9 8.5, MADRS 16.5). Similarly, all RCTs demonstrated improvement in the mean depression scores in both intervention and comparator groups when compared to baseline depression scores at any of the follow-up points [40,41,42, Dekker 2010, Dekker 2011] (Table 2).
Immediately after completion of the main CBT phase, there was a greater improvement in depression for the CBT group compared to the usual care group for BDI-II scores (SMD −0.35, 95% CI −0.63 to −0.07, p = 0.01, I 2 = 0%) and across all depression scales (SMD −0.34, 95% CI −0.60 to −0.08, p = 0.01, I 2 = 0%) (Fig. 3a). Three months after the main CBT phase, there was still a greater improvement in the CBT group than in the usual care group for BDI-II scores (SMD −0.30, 95% CI −0.61 to −0.00, p = 0.05, I 2 = 0%) and across all depression scales (SMD −0.32, 95% CI −0.59 to −0.04, p = 0.03, I 2 = 0%) (Fig. 3b). This demonstrates a moderate size effect initially after the main CBT phase which was maintained 3 months later, and there was no evidence of heterogeneity (I 2 = 0%). For the one study [42] that compared CBT to exercise, there was no difference in depression at 3 months for HAM-D scores (MD −0.34, 95% CI −4.93 to 4.24).
Quality of life
Quality of life was only assessed in the RCTs. Four studies used the Minnesota Living with Heart Failure Questionnaire (MLHFQ) [40, 42, Dekker 2010, Dekker 2011], whilst Freedland et al. [41] used both the Kansas City Cardiomyopathy Questionnaire (KCCQ) and the Short Form 12-item (SF-12). There were improvements in quality of life scores for the CBT intervention groups across all five RCTs, when comparing baseline scores to any of the follow-up points. Immediately after the main CBT phase, there was no difference between the CBT group and usual care group for MLHFQ scores (SMD −0.25, 95% CI −0.68 to 0.18, p = 0.26, I 2 = 52%) (data not shown). However, when all QoL data were combined, there was a greater improvement in the CBT group than the usual care group (SMD −0.31, 95% CI −0.58 to −0.05, p = 0.02, I 2 = 38%) (Fig. 4a), although there was moderate heterogeneity (I 2 values of 38 and 52%, respectively). At 3 months, there were no differences in QoL between the CBT and the usual care groups for either MLHFQ scores (SMD −0.13, 95% CI −0.58 to 0.33, p = 0.58, I 2 = 0%) or across all QoL scales (SMD −0.22, 95% CI −0.51 to 0.06, p = 0.12, I 2 = 0%) (Fig. 4b). One study that compared CBT to exercise demonstrated no difference in QoL between the groups at 3 months (MD −4.23, 95% CI −22.24 to 13.78).
Clinical outcomes
There were limited data on clinical outcomes, and hence, we have limited power for comparison. Four studies provided data on all-cause mortality [40, 42, Dekker 2010, Dekker 2011], with 8/69 (11.6%) deaths in CBT and 7/66 (10.6%) deaths in usual care. There was no difference in all-cause mortality and no evidence of heterogeneity (risk ratio 1.05, 95% CI 0.44 to 2.52, p = 0.63, I 2 = 0%).
Hospitalisations were reported in four studies [40, 41, Dekker 2010, Dekker 2011]. Overall, 55/129 (42.6%) and 55/128 (43.0%) patients in the CBT and usual care groups were hospitalised, respectively, with no difference between the two groups and no evidence of heterogeneity (risk ratio 0.99, 95% CI 0.75 to 1.32, p = 0.96, I 2 = 0%).
Discussion
This systematic review and meta-analysis suggests that CBT may be more effective than usual care at improving depression in heart failure patients initially after the CBT sessions. This difference was sustained 3 months after completion of the CBT sessions; however, these were highly selected patients in selected centres with varied comparators and subjective outcome measures (depression and QoL). The improvement in depression scores evident at 3 months was greater in two RCTs [41, 42]. This may be due to the frequency and duration of the CBT, which were weekly and over a period of time [41, 42], as opposed to a single CBT session [40, Dekker 2010, Dekker 2011]. For quality of life, CBT showed a greater improvement when compared to usual care initially after the main CBT phase; however, there was no evidence of a difference in QoL between the two groups at subsequent time-points. There was no evidence of CBT having an effect on either hospitalisation or mortality.
This systematic review was conducted to evaluate the effects of CBT on depression, quality of life, hospitalisation and mortality in heart failure patients. Previously, there have been two systematic reviews [3, 24] that evaluated the effects of psychological interventions on depression in heart failure patients. However, the first, a 2005 Cochrane review on psychological interventions for depression in heart failure [3], found no relevant RCTs, highlighting the need for RCTs on psychological interventions to be conducted. The second, a 2012 systematic review on the effects of interventions on depression in heart failure, only identified one RCT with a CBT intervention, concluding that there was insufficient evidence on the effects of CBT [24]. Therefore, the emergence of new RCTs, and the lack of conclusive evidence from previous systematic reviews on this topic, justifies the need for the current systematic review to evaluate the effects of CBT in heart failure patients.
Despite the findings of this systematic review, there are several limitations that need to be acknowledged. The searches identified only five RCTs, which demonstrate the lack of experimental studies assessing the effects of CBT in heart failure patients. Two of the RCTs had small sample sizes (≤30), which were particularly problematic with longer follow-up due to attrition. There was also a lack of studies that evaluated the effects of CBT with follow-up ≥6 months, which would have been useful in assessing the long-term sustainability of the effects of CBT. Overall, the methodological quality of studies was unclear due to insufficient information provided. For two studies, only the original data and study protocols could be accessed [Dekker 2010; Dekker 2011], which led to a lack of clarity over certain risks of biases. There was performance bias in the RCTs due to a lack of blinding of participants, but this was unavoidable due to the nature of CBT interventions. Two outcome measures (depression and QoL) were subjective and assessed by self-reported questionnaires, which may have introduced social desirability bias. Data on hospitalisations and deaths were limited and were not reported in all studies. There was also insufficient data to determine the relative effects of CBT in comparison to exercise for depression in heart failure patients, as there was only one RCT that utilised exercise as a comparator. One advantage of this review, however, was that additional data was obtained by contacting lead authors to better inform the systematic review and enable the meta-analysis. Future RCTs on the effects of CBT for heart failure patients would benefit from recruiting larger numbers of participants, delivering weekly CBT sessions over a longer period of time, with long-term follow-up (≥6 months), and reporting on outcomes such as hospitalisations or mortality.
Current NICE guidelines on chronic heart failure [45] state that depression should be treated in accordance with the guidelines for adults with depression [22] and those for adults with a chronic physical health problem [46]; however, the evidence used to evaluate the effects of CBT on depression are not specific to heart failure patients. This may be due to the lack of randomised controlled trials on CBT for depression in heart failure prior to the publication of these guidelines. The 2016 Scottish Intercollegiate Guidelines Network (SIGN) guidelines on the management of chronic heart failure offer a conditional recommendation that CBT should be considered in heart failure patients with depression [47]. The evidence for this recommendation is based on a single RCT on CBT and a systematic review on interventions [24] that included only a single CBT intervention study. Therefore, the current systematic review and meta-analysis extends previous work and provides a comprehensive review of available evidence for the effects of CBT for depression in heart failure patients. The findings from this research have identified an area of further study in heart failure and demonstrated the potential of CBT in selected research centres.
Conclusion
CBT may be more effective than usual care at improving depression in heart failure patients initially after the main CBT phase and 3 months afterwards. CBT also appears to be more effective than usual care at improving quality of life in heart failure patients initially after the main CBT phase, but this effect was not sustained 3 months later. There were no observable differences between CBT and usual care for hospitalisations or mortality. However, these findings were limited by the small sample sizes in some studies, lack of long-term follow-up, use of subjective outcome measures and insufficient information to assess methodological quality. Larger and more robust RCTs are needed to ascertain the long-term benefits and cost-effectiveness of a CBT intervention for depression in heart failure patients.
References
Tendera M (2005) Epidemiology, treatment, and guidelines for the treatment of heart failure in Europe. Eur Heart J 7:J5–J9
McMurray JJV, Stewart S (2002) The burden of heart failure. Eur Heart J 4:D50–D58
Lane DA, Chong AY, Lip GY. Psychological interventions for depression in heart failure (review). Cochrane Database Syst Rev 2005;CD003329
Petersen S, Rayner M, Wolstenholme J (2002) Coronary heart disease statistics: heart failure supplement. British Heart Foundation, London
Stewart S, Macintyre K, Capewell S, McMurrary JJ (2003) Heart failure and the aging population: an increasing burden in the 21st century? Heart 89:49–53
Davis RC, Hobbs FDR, Lip GYH (2000) ABC of heart failure: history and epidemiology. BMJ 320:39–42
Stewart S, Horowitz JD (2002) Home-based intervention in congestive heart failure: long-term implications on readmission and survival. Circulation 105:2861–2866
Royal College of Physicians. Chronic heart failure: the management of adults with chronic heart failure in primary and secondary care (partial update). https://www.nice.org.uk/guidance/CG108/documents/chronic-heart-failure-partial-update-full-guideline2. Accessed 3 Apr 2016
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders: DSM-5, 5th edn. American Psychiatric Association, Washington DC
Vaccarino V, Kasl SV, Abramson J, Krumholz HM (2001) Depressive symptoms and risk of functional decline and death in patients with heart failure. J Am Coll Cardiol 38:199–205
Pihl E, Jacobsson A, Fridlund B, Stromberg A, Martensson J (2005) Depression and health-related quality of life in elderly patients suffering from heart failure and their spouses: a comparative study. Eur J Heart Fail 7:583–589
Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ (2006) Depression in heart failure: a meta-analytic review of prevalence, intervention effects and associations with clinical outcomes. J Am Coll Cardiol 48:1527–1537
Faris R, Purcell H, Henein MY, Coats AJ (2002) Clinical depression is common and significantly associated with reduced survival in patients with non-ischaemic heart failure. Eur J Heart Fail 4:541–551
Jiang W, Kuchibhatla M, Cuffe MS, Christopher EJ, Alexander JD, Clary GL, Blazing MA, Gaulden LH, Califf RM, Krishnan RR, O’Connor CM (2004) Prognostic value of anxiety and depression in patients with chronic heart failure. Circulation 110:3452–3456
Junger J, Schellberg D, Mullter-Tasch T, Raupp G, Zuqck C, Haunstetter A, Zipfel S, Herzog W, Haass M (2005) Depression increasingly predicts mortality in the course of congestive heart failure. Eur J Heart Fail 7:261–267
Kop WJ, Synowski SJ, Gottlieb SS (2011) Depression in heart failure: biobehavioral mechanisms. Heart Failure Clin 7:23–28
Nair N, Farmer C, Gongora E, Dehmer GJ (2012) Commonality between depression and heart failure. Am J Cardiol 109:768–772
Brotman DJ, Golden SH, Wittstein IS (2007) The cardiovascular toll of stress. Lancet 37:1089–1100
Van Der Wal MH, Jaarsma T, Jaarsma T, Moser DK, Veeger NJ, Van Gilst WH, Van Veidhuisen DJ (2006) Compliance in heart failure patients: the importance of knowledge and beliefs. Eur J Heart Fail 27:434–440
Wright M. Cognitive and behavioural therapies. http://patient.info/doctor/cognitive-and-behavioural-therapies. Accessed 3 Apr 2016
Martinez-Devesa P, Perera R, Theodoulou M, Waddell A (2010) Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev 9. doi:10.1002/14651858.CD005233.pub3
NICE. Depression in adults: recognition and management. https://www.nice.org.uk/guidance/cg90/resources/depression-in-adults-recognition-and-management-975742636741. Accessed 3 Apr 2016
Dekker RL (2011) Cognitive therapy for depression in patients with heart failure: a critical review. Heart Fail Clin 7:127–141
Woltz PC, Chapa DW, Friedmann E, Son H, Akintade B, Thomas SA (2012) Effects of interventions on depression in heart failure: a systematic review. Heart Lung 41:469–483
Lane DA, Jeyanantham K, Kotecha D (2016) The effects of cognitive behavioural therapy for depression in heart failure patients. Systematic review and meta-analysis. PROSPERO register. http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016036146. Accessed 7 Mar 2016
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ 338:b2700
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods Group, Cochrane Statistical Methods Group (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343. doi:10.1136/bmj.d5928
Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS, Hahn S, Jang BH, Son HJ (2013) Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol 66:408–414
The Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions, version 5.0.2. http://handbook.cochrane.org/. Accessed 4 Apr 2016
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Cockayne S, Pattenden J, Worthy G, Richardson G, Lewin R (2014) Nurse facilitated self-management support for people with heart failure and their family carers (SEMAPHFOR): a randomised controlled trial. Int J Nurs Stud 51:1207–1213
Smeulders ES, Van Haastregt JC, Ambergen T, Uzko-Lencer NH, Janssen-Boyne JJ, Gorgels AP, Stoffers HE, Lodwewijks-van der Bolt CL, Van Eijk JT, Kempen GI (2010) Nurse-led self-management group programme for patients with congestive heart failure: randomized controlled trial. J Adv Nurs 66:1487–1499
Redeker NS, Jeon S, Andrews L, Cline J, Jacoby D, Mohsenin V (2015) Feasibility and efficacy of a self-management intervention for insomnia in stable heart failure. J Clin Sleep Med 11:1109–1119
Redeker NS, Jeon S, Andrews L, Cline J, Pacelli J, Jacoby D (2013) Cognitive behavioural therapy for insomnia has sustained effects on daytime symptoms and hospitalisation in patients with stable heart failure. Sleep 36:A297
Cully JA, Stanley MA, Deswal A, Hanania NA, Phillips LL, Kunik ME (2010) Cognitive-behavioral therapy for chronic cardiopulmonary conditions: preliminary outcomes from an open trial. Prim Care Companion J Clin Psychiatry 12. doi:10.4088/PCC.09m00896blu
Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, Czajkowski SM, DeBusk R, Hosking J, Jaffe A, Kaufmann PG, Mitchell P, Normal J, Powell LH, Raczynski JM, Schneiddermann N, Enhancing Recovery in Coronary Heart Disease Patients Investigations (ENRICHD) (2003) Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the enhancing recovery in coronary heart disease patients (ENRICHD) randomized trial. JAMA 289:3106–3116
Carney RM, Freedland KE, Steinmeyer B, Rubin EH, Mann DL, Rich MW (2016) Cardiac risk markers and response to depression treatment in patients with coronary heart disease. Psychosom Med 78:49–59
Moser DK, Wu KR, Chung ML, Biddle MJ, Schooler M, Lennie TA (2012) Randomized controlled trial of a biobehavioral intervention for depression in patients with heart failure. J Card Fail 1:S69
Chung ML, Lennie TA, Moser DK (2014) The feasibility of the family cognitive educational intervention to improve depressive symptoms and quality of life in patients with heart failure and their family caregivers. J Card Fail 1:S52
Dekker RL, Moser DK, Peden AR, Lennie TA (2012) Cognitive therapy improves three-month outcomes in hospitalized patients with heart failure. J Card Fail 18:19–20
Freedland KE, Carney RM, Rich MW, Steinmeyer BC, Rubin EH (2015) Cognitive behavior therapy for depression and self-care in heart failure patients: a randomized clinical trial. JAMA Intern Med 175:1773–1782
Gary RA, Dunbar SB, Higgins MK, Musselman DL, Smith AL (2010) Combined exercise and cognitive behavioral therapy improves outcomes in patients with heart failure. J Psychosom Res 69:119–131
Lundgren J, Andersson G, Dahlström Ö, Jaarsma T, Köhler AK, Johansson P (2015) Internet-based cognitive behavior therapy for patients with heart failure and depressive symptoms: a proof of concept study. Patient Educ Couns 98:935–942
Dekker RL, Tovar EG, Doering LV, Bailey AL, Campbell CL, Wright JH, Bishop ML, Moser DK (2014) A single cognitive behavior therapy session improves short-term depressive symptoms in hospitalized patients with heart failure. Circulation 130:A13961
NICE. Chronic heart failure in adults: management. https://www.nice.org.uk/guidance/cg108. Accessed 4 Apr 2016
NICE. Depression in adults with a chronic physical health problem: recognition and management. https://www.nice.org.uk/guidance/cg91. Accessed 4 Apr 2016
SIGN. Management of chronic heart failure. http://www.sign.ac.uk/pdf/SIGN147.pdf. Accessed 4 Apr 2016
Acknowledgements
The authors would like to thank Professor Rebecca Gary and Professor Melinda Higgins for providing additional data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The Bachelor of Medical Science (BMedSc) Population Sciences and Humanities (PoSH) programme at the University of Birmingham, UK funded Mr. Kishaan Jeyanantham to conduct this systematic review. Dr. Kotecha is funded by a UK National Institute of Health Research (NIHR) Career Development Fellowship (CDF-2015-08-074). Dr. Dekker’s work was supported by the National Institutes of Health, National Institute of Nursing Research [K23 NR013480]; the University of Kentucky College of Nursing Center for Biobehavioral Research on Self-Management [NINR, P20 NR 010679]; the University of Kentucky Center for Clinical and Translational Sciences [NIH CTSA UL1TR000117]; and a University of Kentucky Faculty Research Support Grant. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research, the National Institutes of Health or NIHR.
Conflicts of interest
Mr. Jeyanantham and Mr. Thanki have no conflicts of interest or financial ties to disclose. Dr. Kotecha has received research grants from Menarini, lecture fees from AtriCure, professional development support from Daiichi Sankyo and is the lead for the Beta-blockers in Heart Failure Collaborative Group (BB-meta-HF) and the RAte-control Therapy Evaluation in Atrial Fibrillation trial group (RATE-AF). Professor Dekker has no conflicts of interest or financial ties to disclose. Dr. Lane has received investigator-initiated educational grants from Bristol Myers Squibb and Boehringer Ingelheim, and has been a speaker and consultant for Boehringer Ingelheim, Bayer, and Bristol Myers Squibb/Pfizer.
Ethical approval
This article is a systematic review and meta-analysis of published data and therefore does not contain any studies with human participants performed by any of the authors. The original studies on which this systematic review is based will have obtained informed consent from all participants included in the study.
Electronic supplementary material
Supplementary Table 1
(DOCX 24 kb).
Supplementary Table 2
(DOCX 23 kb).
Supplementary Table 3
(DOCX 26 kb).
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jeyanantham, K., Kotecha, D., Thanki, D. et al. Effects of cognitive behavioural therapy for depression in heart failure patients: a systematic review and meta-analysis. Heart Fail Rev 22, 731–741 (2017). https://doi.org/10.1007/s10741-017-9640-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-017-9640-5