Abstract
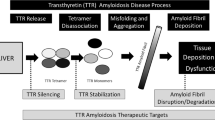
Transthyretin-cardiac amyloidoses (ATTR-CA) are an underdiagnosed but increasingly recognized cause of heart failure. Extracellular deposition of fibrillary proteins into tissues due to a variety of inherited transthyretin mutations in ATTRm or due to advanced age in ATTRwt eventually leads to organ failure. In the heart, amyloid deposition causes diastolic dysfunction, restrictive cardiomyopathy with progressive loss of systolic function, arrhythmias, and heart failure. While traditional treatments have consisted of conventional heart failure management and supportive care for systemic symptoms, numerous disease-modifying therapies have emerged over the past decade. From organ transplantation to transthyretin stabilizers (diflunisal, tafamidis, AG-1), TTR silencers (ALN-ATTR02, ISIS-TTR(Rx)), and degraders of amyloid fibrils (doxycycline/TUDCA), the potential for effective transthyretin amyloid therapy is greater now than ever before. In light of these multiple agents under investigation in human clinical trials, clinicians should be familiar with the systemic cardiac amyloidoses, their differing pathophysiology, natural histories, and unique treatment strategies.



Similar content being viewed by others
References
Dharmarajan K, Maurer MS (2012) Transthyretin cardiac amyloidoses in older North Americans. J Am Geriatr Soc 60(4):765–774
Rapezzi C et al (2009) Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation 120(13):1203–1212
Connors LH et al (2009) Cardiac amyloidosis in African Americans: comparison of clinical and laboratory features of transthyretin V122I amyloidosis and immunoglobulin light chain amyloidosis. Am Heart J 158(4):607–614
Jacobson DR et al (1996) Revised transthyretin Ile 122 allele frequency in African-Americans. Hum Genet 98(2):236–238
Hornsten R et al (2010) Heart complications in familial transthyretin amyloidosis: impact of age and gender. Amyloid 17(2):63–68
Bonaiti B et al (2010) TTR familial amyloid polyneuropathy: does a mitochondrial polymorphism entirely explain the parent-of-origin difference in penetrance? Eur J Hum Genet 18(8):948–952
Rapezzi C et al (2008) Gender-related risk of myocardial involvement in systemic amyloidosis. Amyloid 15(1):40–48
Suhr OB et al (2006) Myocardial hypertrophy and function are related to age at onset in familial amyloidotic polyneuropathy. Amyloid 13(3):154–159
Suhr O et al (1994) Malnutrition and gastrointestinal dysfunction as prognostic factors for survival in familial amyloidotic polyneuropathy. J Intern Med 235(5):479–485
Coelho T, Maurer MS, Suhr OB (2013) THAOS—the transthyretin amyloidosis outcomes survey: initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr Med Res Opin 29(1):63–76
Maurer MS et al (2013) Cardiac biomarkers in patients with transthyretin amyloidosis as documented in THAOS: the transthyretin amyloidosis survey. J Am Coll Cardiol 61(10):E1244–E1244
Givens RC et al (2013) Comparison of cardiac amyloidosis due to wild-type and V122I transthyretin in older adults referred to an academic medical center. Aging Health 9(2):229–235
Ihse E et al (2008) Amyloid fibril composition is related to the phenotype of hereditary transthyretin V30M amyloidosis. J Pathol 216(2):253–261
Khella S, Drachman B, Divito P, Polydefkis M, Brannagan T, Judge D, Maurer MS (2013) Neurologic involvement in Val122Ile familial amyloidosis. In: IXth international symposium on familial amyloid polyneuropathy; VIIIth international symposium on liver transplantation in familial amyloid polyneuropathy, Rio de Janeiro, Brazil
Ng B et al (2005) Senile systemic amyloidosis presenting with heart failure: a comparison with light chain-associated amyloidosis. Arch Intern Med 165(12):1425–1429
Pinney JH et al (2013) Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc 2(2):e000098
Quarta CC et al (2014) Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation 129(18):1840–1849
Liao R et al (2001) Infusion of light chains from patients with cardiac amyloidosis causes diastolic dysfunction in isolated mouse hearts. Circulation 104(14):1594–1597
Mishra S et al (2013) Human amyloidogenic light chain proteins result in cardiac dysfunction, cell death, and early mortality in zebrafish. Am J Physiol Heart Circ Physiol 305(1):H95–H103
Ruberg FL et al (2012) Prospective evaluation of the morbidity and mortality of wild-type and V122I mutant transthyretin amyloid cardiomyopathy: the transthyretin amyloidosis cardiac study (TRACS). Am Heart J 164(2):222–228 e1
Falk RH (2005) Diagnosis and management of the cardiac amyloidoses. Circulation 112(13):2047–2060
Feng D et al (2007) Intracardiac thrombosis and embolism in patients with cardiac amyloidosis. Circulation 116(21):2420–2426
Berghoff M et al (2003) Endothelial dysfunction precedes C-fiber abnormalities in primary (AL) amyloidosis. Ann Neurol 53(6):725–730
Yood RA et al (1983) Bleeding manifestations in 100 patients with amyloidosis. JAMA 249(10):1322–1324
Feng D et al (2009) Intracardiac thrombosis and anticoagulation therapy in cardiac amyloidosis. Circulation 119(18):2490–2497
Dubrey S et al (1995) Atrial thrombi occurring during sinus rhythm in cardiac amyloidosis: evidence for atrial electromechanical dissociation. Br Heart J 74(5):541–544
Murphy L, Falk RH (2000) Left atrial kinetic energy in AL amyloidosis: can it detect early dysfunction? Am J Cardiol 86(2):244–246
Lip GY et al (2010) Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro heart survey on atrial fibrillation. Chest 137(2):263–272
Klein AL et al (1990) Comprehensive doppler assessment of right ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol 15(1):99–108
Koyama J, Ray-Sequin PA, Falk RH (2003) Longitudinal myocardial function assessed by tissue velocity, strain, and strain rate tissue doppler echocardiography in patients with AL (primary) cardiac amyloidosis. Circulation 107(19):2446–2452
King DL, El-Khoury Coffin L, Maurer MS (2002) Myocardial contraction fraction: a volumetric index of myocardial shortening by freehand three-dimensional echocardiography. J Am Coll Cardiol 40(2):325–329
Tendler AHA, Maurer MS (2013) Myocardial contraction fraction is superior to the myocardial contraction fraction is superior to ejection fraction in predicting survival in patients with cardiac amyloidosis. In: IX international symposium on familial amyloidotic polyneuropathy (ISFAP) and the VIII international symposium on liver transplantation in familial amyloidotic polyneuropathy, Rio de Janeiro, Brazil
Damy T, Plante-Bordeneuve V, Karayal O, Mundayat R, Kristen AV (2013) Clinical and echocardiographic signs associated with increased interventricular thickness (IVST) due to TTR related amyloidosis. In: European Society of Cardiology, Amsterdam, Netherlands
Suhr OB et al (2000) Liver transplantation for hereditary transthyretin amyloidosis. Liver Transpl 6(3):263–276
Tsay DM, Maurer MS (2014) Biomarkers in ATTR cardiac amyloidosis (in submission)
Bokhari S et al (2013) (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging 6(2):195–201
Cassidy JT (1961) Cardiac amyloidosis. Two cases with digitalis sensitivity. Ann Intern Med 55:989–994
Pomerance A (1965) Senile cardiac amyloidosis. Br Heart J 27(5):711–718
Rubinow A, Skinner M, Cohen AS (1981) Digoxin sensitivity in amyloid cardiomyopathy. Circulation 63(6):1285–1288
Wiklund U et al (2008) Abnormal heart rate variability and subtle atrial arrhythmia in patients with familial amyloidotic polyneuropathy. Ann Noninvasive Electrocardiol 13(3):249–256
Okamoto S et al (2011) Continuous development of arrhythmia is observed in Swedish transplant patients with familial amyloidotic polyneuropathy (amyloidogenic transthyretin Val30Met variant). Liver Transpl 17(2):122–128
Algalarrondo V et al (2012) Prophylactic pacemaker implantation in familial amyloid polyneuropathy. Heart Rhythm 9(7):1069–1075
Epstein AE et al (2008) ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: executive summary. Heart Rhythm 5(6):934–955
Zhao Y et al (2012) Left ventricular dyssynchrony is associated with reduced heart rate variability in familial amyloidotic polyneuropathy. Int J Cardiol 155(2):273–278
Dubrey SW et al (1998) The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM 91(2):141–157
Falk RH, Rubinow A, Cohen AS (1984) Cardiac arrhythmias in systemic amyloidosis: correlation with echocardiographic abnormalities. J Am Coll Cardiol 3(1):107–113
Kristen AV et al (2008) Prophylactic implantation of cardioverter-defibrillator in patients with severe cardiac amyloidosis and high risk for sudden cardiac death. Heart Rhythm 5(2):235–240
Dhoble A et al (2009) Cardiac amyloidosis treated with an implantable cardioverter defibrillator and subcutaneous array lead system: report of a case and literature review. Clin Cardiol 32(8):E63–E65
Lin G, Dispenzieri A, Brady PA (2010) Successful termination of a ventricular arrhythmia by implantable cardioverter defibrillator therapy in a patient with cardiac amyloidosis: insight into mechanisms of sudden death. Eur Heart J 31(12):1538
Lin G et al (2013) Implantable cardioverter defibrillators in patients with cardiac amyloidosis. J Cardiovasc Electrophysiol 24(7):793–798
Garan AR, Kolluri S, Lombardo I, Maurer MS (2012) The prevalence of Holter abnormalities in ATTR cardiac amyloidosis. In: XIII international symposium on amyloidosis, Groningen, Netherlands
Varr BC et al (2014) Implantable cardioverter-defibrillator placement in patients with cardiac amyloidosis. Heart Rhythm 11(1):158–162
Holmgren G et al (1991) Biochemical effect of liver transplantation in two Swedish patients with familial amyloidotic polyneuropathy (FAP-met30). Clin Genet 40(3):242–246
Holmgren G et al (1993) Clinical improvement and amyloid regression after liver transplantation in hereditary transthyretin amyloidosis. Lancet 341(8853):1113–1116
Jonsen E et al (2001) Early liver transplantation is essential for familial amyloidotic polyneuropathy patients’ quality of life. Amyloid 8(1):52–57
Sandgren O et al (1991) Vitreous involvement in familial amyloidotic neuropathy: a genealogical and genetic study. Clin Genet 40(6):452–460
Olofsson BO (1983) Cardiac involvement in familial amyloidosis with polyneuropathy. Int J Cardiol 4(3):379–382
Steen LE, Ek BO (1984) Familial amyloidosis with polyneuropathy. Aspects of the relationship between gastrointestinal symptoms, EMG findings, and malabsorption studies. Scand J Gastroenterol 19(4):480–486
Bispo M et al (2009) High incidence of thrombotic complications early after liver transplantation for familial amyloidotic polyneuropathy. Transpl Int 22(2):165–171
Sharma P et al (2003) Outcome of liver transplantation for familial amyloidotic polyneuropathy. Liver Transpl 9(12):1273–1280
Liepnieks JJ, Benson MD (2007) Progression of cardiac amyloid deposition in hereditary transthyretin amyloidosis patients after liver transplantation. Amyloid 14(4):277–282
Liepnieks JJ, Zhang LQ, Benson MD (2010) Progression of transthyretin amyloid neuropathy after liver transplantation. Neurology 75(4):324–327
Gustafsson S et al (2012) Amyloid fibril composition as a predictor of development of cardiomyopathy after liver transplantation for hereditary transthyretin amyloidosis. Transplantation 93(10):1017–1023
Furtado A et al (1997) Sequential liver transplantation. Transplant Proc 29(1–2):467–468
Furtado L et al (1999) Maximum sharing of cadaver liver grafts composite split and domino liver transplants. Liver Transpl Surg 5(2):157–158
Tome L et al (2001) Sequential liver transplantation: 27 cases in 25 patients. Transplant Proc 33(1–2):1430–1432
Bolte FJ et al (2013) Evaluation of domino liver transplantations in Germany. Transpl Int 26(7):715–723
Abdelfatah MM, Hayman SR, Gertz MA (2014) Domino liver transplantation as a cause of acquired familial amyloid polyneuropathy. Amyloid 21(2):136–137
Conner R et al (1988) Heart transplantation for cardiac amyloidosis: successful one-year outcome despite recurrence of the disease. J Heart Transplant 7(2):165–167
Kpodonu J et al (2005) Outcome of heart transplantation in patients with amyloid cardiomyopathy. J Heart Lung Transplant 24(11):1763–1765
Rapezzi C et al (2010) Transthyretin-related amyloidoses and the heart: a clinical overview. Nat Rev Cardiol 7(7):398–408
Sekijima Y, Dendle MA, Kelly JW (2006) Orally administered diflunisal stabilizes transthyretin against dissociation required for amyloidogenesis. Amyloid 13(4):236–249
Tojo K et al (2006) Diflunisal stabilizes familial amyloid polyneuropathy-associated transthyretin variant tetramers in serum against dissociation required for amyloidogenesis. Neurosci Res 56(4):441–449
Johnson SM et al (2012) The transthyretin amyloidoses: from delineating the molecular mechanism of aggregation linked to pathology to a regulatory-agency-approved drug. J Mol Biol 421(2–3):185–203
Miller SR, Sekijima Y, Kelly JW (2004) Native state stabilization by NSAIDs inhibits transthyretin amyloidogenesis from the most common familial disease variants. Lab Invest 84(5):545–552
Adamski-Werner SL et al (2004) Diflunisal analogues stabilize the native state of transthyretin. Potent inhibition of amyloidogenesis. J Med Chem 47(2):355–374
Kearney PM et al (2006) Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ 332(7553):1302–1308
Epstein M (2002) Non-steroidal anti-inflammatory drugs and the continuum of renal dysfunction. J Hypertens Suppl 20(6):S17–S23
Marnett LJ, Kalgutkar AS (1999) Cyclooxygenase 2 inhibitors: discovery, selectivity and the future. Trends Pharmacol Sci 20(11):465–469
Wallace JL (2001) Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol 15(5):691–703
Mukherjee D, Nissen SE, Topol EJ (2001) Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 286(8):954–959
Page J, Henry D (2000) Consumption of NSAIDs and the development of congestive heart failure in elderly patients: an underrecognized public health problem. Arch Intern Med 160(6):777–784
Castano A et al (2012) Diflunisal for ATTR cardiac amyloidosis. Congest Heart Fail 18(6):315–319
Berk JL et al (2013) Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA 310(24):2658–2667
Quarta CCF, Solomon RH, Suhr SD, Obici OB, Perlini L, Lindqvist S, Koyama P, Sekijima J, Zeldenrust Y, Yamashita SR, Horibata T, Miller Y, Gorevic F, Merlini P, Ando G, Ikeda Y, Ruberg S, Berk F (2014) The prevalence of cardiac amyloidosis in familial amyloidotic polyneuropathy with predominant neuropathy: the diflunisal trial. In: International symposium on amyloidosis, Indianapolis, USA, pp 88–89
Bulawa CE et al (2012) Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci U S A 109(24):9629–9634
Hammarstrom P et al (2002) Sequence-dependent denaturation energetics: a major determinant in amyloid disease diversity. Proc Natl Acad Sci USA 99(Suppl 4):16427–16432
Coelho T et al (2012) Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology 79(8):785–792
Merlini G et al (2013) Effects of tafamidis on transthyretin stabilization and clinical outcomes in patients with non-Val30Met transthyretin amyloidosis. J Cardiovasc Transl Res 6(6):1011–1020
Maurer MS, JDP, Rosas GR, Mandel FS, Aarts J (2014) Interim analysis of long-term, open-label tafamidis treatment in transthyretin amyloid cardiomyopathy after up to 5 years of treatment. In: International symposium on amyloidosis, Indianapolis, USA
Pfizer (2014) Safety and efficacy of tafamidis in patients with transthyretin cardiomyopathy (ATTR-ACT). Available from: http://www.clinicaltrials.gov/show/NCT01994889
Alhamadsheh MM et al (2011) Potent kinetic stabilizers that prevent transthyretin-mediated cardiomyocyte proteotoxicity. Sci Transl Med 3(97):97ra81
Penchala SC et al (2013) AG10 inhibits amyloidogenesis and cellular toxicity of the familial amyloid cardiomyopathy-associated V122I transthyretin. Proc Natl Acad Sci USA 110(24):9992–9997
Fire A et al (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391(6669):806–811
Elbashir SM et al (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411(6836):494–498
Delivery, A.R.R.C. (2012) Available from: http://www.alnylam.com/capella/roundtables/rnai-roundtable-conjugate-delivery-dec-14/
Kanasty R et al (2013) Delivery materials for siRNA therapeutics. Nat Mater 12(11):967–977
Coelho T et al (2013) Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med 369(9):819–829
van Bennekum AM et al (2001) Biochemical basis for depressed serum retinol levels in transthyretin-deficient mice. J Biol Chem 276(2):1107–1113
Pharmaceuticals A (2014) Phase 2 study to evaluate ALN-TTRSC in patients with transthyretin (TTR) cardiac amyloidosis
Benson MD et al (2006) Targeted suppression of an amyloidogenic transthyretin with antisense oligonucleotides. Muscle Nerve 33(5):609–618
Benson MD et al (2011) Antisense oligonucleotide therapy for TTR amyloidosis. Amyloid 18(Suppl 1):60
Ackermann EJ et al (2012) Clinical development of an antisense therapy for the treatment of transthyretin-associated polyneuropathy. Amyloid 19(Suppl 1):43–44
Pharmaceuticals, I (2013) Efficacy and safety of ISIS-TTRRx in familial amyloid polyneuropathy. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01737398?term=ISIS+amyloid&rank=1
Cardoso I, Saraiva MJ (2006) Doxycycline disrupts transthyretin amyloid: evidence from studies in a FAP transgenic mice model. FASEB J 20(2):234–239
Cardoso I et al (2010) Synergy of combined doxycycline/TUDCA treatment in lowering transthyretin deposition and associated biomarkers: studies in FAP mouse models. J Transl Med 8:74
Obici L et al (2012) Doxycycline plus tauroursodeoxycholic acid for transthyretin amyloidosis: a phase II study. Amyloid 19(Suppl 1):34–36
Matteo IPS (2011) Safety, efficacy and pharmacokinetics of doxycycline plus tauroursodeoxycholic acid in transthyretin amyloidosis. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01171859?term=%EF%82%A7%09NCT01171859&rank=1
Hospital, B.a.W.s. (2013) Tolerability and efficacy of a combination of doxycycline and TUDCA in patients with transthyretin amyloid cardiomyopathy. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01855360?term=NCT01855360&rank=1
Pepys MB, Dash AC (1977) Isolation of amyloid P component (protein AP) from normal serum as a calcium-dependent binding protein. Lancet 1(8020):1029–1031
Bodin K et al (2010) Antibodies to human serum amyloid P component eliminate visceral amyloid deposits. Nature 468(7320):93–97
Pepys MB et al (2002) Targeted pharmacological depletion of serum amyloid P component for treatment of human amyloidosis. Nature 417(6886):254–259
Acknowledgments
We acknowledge the patients with cardiac amyloidosis who continue to participate in clinical trials and wait patiently for the development of effective treatments.
Conflict of interest
Dr. Castaño has no conflicts of interest or financial ties to disclose. Dr. Drachman has received funding as a scientific advisor to ISIS Pharmaceuticals. Dr. Judge has received funding as a scientific advisor to both Pfizer and Alnylam Pharmaceuticals. Dr. Maurer serves on the advisory board of the Transthyretin Amyloid Outcomes Survey (THAOS), which is funded by Pfizer, Inc., and has received unrestricted educational grant support from Alnylam Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castaño, A., Drachman, B.M., Judge, D. et al. Natural history and therapy of TTR-cardiac amyloidosis: emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev 20, 163–178 (2015). https://doi.org/10.1007/s10741-014-9462-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-014-9462-7