Abstract
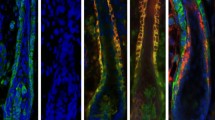
The rat whisker hair follicle (HF) is a model for studying the reconstruction of the HF or dermal papilla (DP), and involves the Wnt/β-catenin signaling pathway, which is a key pathway in HF development and HF cycling after birth. It has been reported that Wnt/catenin signaling plays an indispensable role in human or rat pelages development and postnatal growth. However, the distribution of some Wnt/β-catenin signaling pathway factors and their relationship with the epithelial stem cell markers in whisker follicles has not been characterized. In this study, we investigated the immunolocalization of Wnt/catenin signaling pathway members, including Wnt10b, Wnt10a, Wnt5a, β-catenin, and downstream lymphoid enhancer-binding factor 1 (LEF1) and transcription factor 3 (TCF3), as well as, HF stem-cell markers CD34, CK15 and proliferating cell nuclear antigen (PCNA) protein, in rat anagen phase whisker follicles. β-catenin, Wnt5a, Wnt10b, Wnt10a, LEF1, and TCF3 were expressed in the outer root sheath (ORS), inner root sheath, matrix and hair shaft of anagen follicles. β-catenin, Wnt10b, LEF1, and TCF3 were highly expressed and Wnt5a and Wnt10a weakly expressed in DP and dermal sheath (DS) regions. The expression of α-smooth muscle actin was strong in the lower DS and it was also detected in some DP cells. CD34, CK15 and PCNA were all expressed in the ORS; and CD34 and PCNA were also detected in the matrix, however CD34 was extensively expressed in DP and DS regions. Our studies located the position of Wnts, downstream LEF1 and TCF3 and stem cell marker proteins, which provide new information in understanding the role of the Wnt singaling pathway in whisker follicles’ growth.




Similar content being viewed by others
Abbreviations
- Bu:
-
Bulge
- C:
-
Capsule
- DP:
-
Dermal papilla
- DS:
-
Dermal sheath
- GM:
-
Glassy membrane
- HF:
-
Hair follicle
- HS:
-
Hair shaft
- IRS:
-
Inner root sheath
- Mx:
-
Matrix
- ORS:
-
Outer root sheath
- P:
-
Precortex
References
Alonso L, Fuchs E (2006) The hair cycle. J Cell Sci 119:391–393
Andl T, Reddy ST, Gaddapara T, Millar SE (2002) Wnt signals are required for the initiation of hair follicle development. Dev Cell 2:643–653
Cohen J (1961) The transplantation of individual rat and guineapig whisker papillae. J Embryol Exp Morphol 9:117–127
DasGupta R, Fuchs E (1999) Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126:4557–4568
Enshell-Seijffers D, Lindon C, Kashiwagi M, Morgan B (2010) β-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev Cell 18(4):633–642
Hendaoui I, Tucker RP, Zingg D, Bichet S, Schittny J, Chiquet-Ehrismann R (2014) Tenascin-C is required for normal Wnt/beta-catenin signaling in the whisker follicle stem cell niche. Matrix Biol 40:46–53
Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W (2001) β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 105(4):533–545
Iida M, Ihara S, Matsuzaki T (2007) Follicular epithelia and dermal papillae of mouse vibrissal follicles qualitatively change their hair-forming ability during anagen. Differentiation 75:371–381
Jahoda CA, Reynolds AJ, Chaponnier C, Forester JC, Gabbiani G (1991) Smooth muscle alpha-actin is a marker for hair follicle dermis in vivo and in vitro. J Cell Sci 99:627–636
Kim JC, Choi YC (1995) Regrowth of grafted human scalp hair after removal of the bulb. Dermatol Surg 21:312–313
Kim BK, Lee HY, Kim I, Choi K, Park J, Yoon SK (2014) Increased expression of Dkk1 by HR is associated with alteration of hair cycle in hairpoor mice. J Dermatol Sci 74:81–87
Kobayashi K, Nishimura E (1989) Ectopic growth of mouse whiskers from implanted lengths of plucked vibrissa follicles. J Invest Dermatol 92:278–282
Krause K, Foitzik K (2006) Biology of the hair follicle: the basics. Semin Cutan Med Surg 25:2–10
Larouche D, Tong X, Fradette J, Coulombe PA, Germain L (2008) Vibrissa hair bulge houses two populations of skin epithelial stem cells distinct by their keratin profile. FASEB J 22:1404–1415
Lavker RM, Sun TT (2000) Epidermal stem cells: properties, markers, and location. Proc Natl Acad Sci USA 97:13473–13475
Lavker RM, Cotsarelis G, Wei ZG, Sun TT (1991) Stem cells of pelage, vibrissae, and eyelash follicles: The hair cycle and tumor formation. Ann N Y Acad Sci 642:214–224 (discussion 224–215)
Lavker RM, Sun TT, Oshima H, Barrandon Y, Akiyama M, Ferraris C, Chevalier G, Favier B, Jahoda CA, Dhouailly D, Panteleyev AA, Christiano AM (2003) Hair follicle stem cells. J Investig Dermatol Symp Proc 8:28–38
Li YH, Zhang K, Ye JX, Lian XH, Yang T (2011) Wnt10b promotes growth of hair follicles via a canonical Wnt signalling pathway. Clin Exp Dermatol 36:534–540
Li YH, Zhang K, Yang K, Ye JX, Xing YZ, Guo HY, Deng F, Lian XH, Yang T (2013) Adenovirus-mediated Wnt10b overexpression induces hair follicle regeneration. J Invest Dermatol 133:42–48
Millar SE, Willert K, Salinas PC, Roelink H, Nusse R, Sussman DJ, Barsh GS (1999) Wnt signaling in the control of hair growth and structure. Dev Biol 207:133–149
Morioka K, Arai M, Ihara S (2011) Steady and temporary expressions of smooth muscle actin in hair, vibrissa, arrector pili muscle, and other hair appendages of developing rats. Acta Histochem Cytochem 44:141–153
Myung PS, Takeo M, Ito M, Atit RP (2013) Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J Invest Dermatol 133:31–41
Oliver RF (1966a) Regeneration of dermal papillae in rat vibrissae. J Invest Dermatol 47:496–497
Oliver RF (1966b) Histological studies of whisker regeneration in the hooded rat. J Embryol Exp Morphol 16:231–244
Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE, Millar SE (2001) Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of sonic hedgehog in hair follicle morphogenesis. Mech Dev 107:69–82
Stenn KS, Paus R (2001) Controls of hair follicle cycling. Physiol Rev 81:449–494
Trempus CS, Morris RJ, Bortner CD, Cotsarelis G, Faircloth RS, Reece JM, Tennant RW (2003) Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol 120:501–511
Tucker RP, Ferralli J, Schittny JC, Chiquet-Ehrismann R (2013) Tenascin-c and tenascin-w in whisker follicle stem cell niches: Possible roles in regulating stem cell proliferation and migration. J Cell Sci 126:5111–5115
Wang B, Li H, Liu Y, Lin X, Lin Y, Wang Y, Hu X, Zhang Y (2014) Expression patterns of WNT/beta-CATENIN signaling molecules during human tooth development. J Mol Histol 45(5):487–496
Waters JM, Lindo JE, Arkell RM, Cowin AJ (2011) Regeneration of hair follicles is modulated by flightless I (Flii) in a rodent vibrissa model. J Invest Dermatol 131:838–847
Xing Y, Xu W, Yang K, Lian X, Yang T (2011) Immunolocalization of Wnt5a during the hair cycle and its role in hair shaft growth in mice. Acta Histochem 113:608–612
Xing YZ, Wang RM, Yang K, Guo HY, Deng F, Li YH, Ye JX, He L, Lian XH, Yang T (2013) Adenovirus-mediated Wnt5a expression inhibits the telogen-to-anagen transition of hair follicles in mice. Int J Med Sci 10:908–914
Young RD, Oliver RF (1976) Morphological changes associated with the growth cycle of vibrissal follicles in the rat. J Embryol Exp Morphol 36:597–607
Acknowledgments
This work was supported by grants from the National Science Foundation of China (Nos. 81372084, 81171832) and the Guangdong Province Outstanding Young Teacher Training Program (No. Yq2013078).
Author information
Authors and Affiliations
Corresponding author
Additional information
Chang-min Lin and Yan-ping Yuan have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lin, Cm., Yuan, Yp., Chen, Xc. et al. Expression of Wnt/β-catenin signaling, stem-cell markers and proliferating cell markers in rat whisker hair follicles. J Mol Hist 46, 233–240 (2015). https://doi.org/10.1007/s10735-015-9616-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-015-9616-5