Abstract
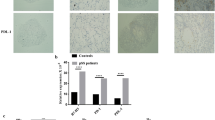
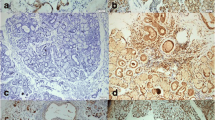
Diagnosis and therapeutic strategies in Sjögren’s syndrome (SS) might greatly benefit of the present multidisciplinary approach to studying the molecular pathogenesis of the disease. A deregulated inflammatory response has been described in the SS. The research in the last years sheds light on the importance of the NF-κB pathway regulating the pro-inflammatory cytokine production and leukocyte recruitment. These are important contributors to the inflammatory response during the development of SS. In this study we examine the expression of the NF-κB inhibitory protein termed IκBα in salivary glands epithelial cells (SGEC) comparing it with SGEC from healthy controls, to test the hypothesis that an altered expression of IκBα occurs in SGEC from SS biopsies. Real-Time PCR, western blot and immunohistochemistry demonstrated that the expression level of IκBα was significantly lower in SS with respect to healthy controls leading to an increased NF-κB activity. Our results suggest that the analysis of IκBα expression at salivary gland epithelial cell level could be a potential new hallmark of SS progression and sustain a rationale to more deeply investigate the therapeutic potential of specific NF-κB inhibitors in SS.


Similar content being viewed by others
References
Azuma M, Motegi K, Aota K, Hayashi Y, Sato M (1997) Role of cytokines in the destruction of acinar structure in Sjogren’s syndrome salivary glands. Lab Invest 77:269–280
Baeuerle PA (1998) Pro-inflammatory signaling: last pieces in the NFkappa B puzzle? Curr Biol 8:R19–R22
Baldwin AS (1996) The NF-kB and IkB proteins: new discoveries and insights. Annu Rev Immunol 14:649–681
Barnes PJ, Karin M (1997) Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 336:1066–1071
Beg AA, Finco TS, Nantermet PV, Baldwin AS (1993) Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of IkB: a mechanism for NF-kB activation. Mol Cell Biol 13:3301–3310
Christman JW, Sadikot RT, Blackwell TS (2000) The role of nuclear factor-kappa B in pulmonary diseases. Chest 117(5):1482–1487
Dale E, Davis M, Faustman DL (2006) A role for transcription factor NF-kappa B in autoimmunity: possible interactions of genes, sex, and the immune response. Adv Physiol Educ 30:152–158
Fox PC (2007) Autoimmune diseases and Sjogren’s syndrome: an autoimmune exocrinopathy. Ann N Y Acad Sci 1098:15–21
Foxwell B, Browne K, Bondeson J, Clarke C, de Martin R, Brennan F, Feldmann M (1998) Efficient adenoviral infection with IkappaB alpha reveals that macrophage tumor necrosis factor alpha production in rheumatoid arthritis is NF-kappaB dependent. Proc Natl Acad Sci USA 95:8211–8215
Gestermann N, Mekinian A, Comets E, Loiseau P, Puechal X, Hachulla E, Gottenberg JE, Mariette X, Miceli-Richard C (2010) STAT4 is a confirmed genetic risk factor for Sjögren’s syndrome and could be involved in type 1 interferon pathway signalling. Genes Immun 11:432–438
Han ZN, Boyle DL, Manning AM, Firestein GS (1998) AP-1 and NF-kappa B regulation in rheumatoid arthritis and murine collagen-induced arthritis. Autoimmunity 28:197–208
Jacobs MD, Harrison SC (1998) Structure of an IkappaBalpha/NF-kappaB complex. Cell 95:749–758
Kapsogeorgou EK, Dimitriou ID, Abu-Helu RF, Moutsopoulos HM, Manoussakis MN (2001) Activation of epithelial and myoepithelial cells in the salivary glands of patients with Sjögren’s syndrome: high expression of intercellular adhesion molecule-1 (ICAM.1) in biopsy specimens and cultured cells. Clin Exp Immunol 124:126–133
Kittridge A, Routhouska SB, Korman NJ (2011) Dermatologic manifestations of Sjögren syndrome. J Cutan Med Surg 15(1):8–14
Klein W, Tromm A, Folwaczny C, Hagedorn M, Duerig N, Epplen JT, Schmiegel WH, Griga T (2004) A polymorphism of the NFKBIA gene is associated with Crohn’s disease patients lacking a predisposing allele of the CARD15 gene. Int J Colorectal Dis 19(2):153–156
Klement JF, Rice NR, Car BD, Abbondanzo SJ, Powers GD, Bhatt PH, Chen CH, Rosen CA, Stewart CL (1996) IkappaBalpha deficiency results in a sustained NF-kappaB response and severe widespread dermatitis in mice. Mol Cell Biol 16:2341–2349
Li Q, Verma IM (2002) NF-kappa B regulation in the immune system. Nat Rev Immunol 2:725–734
Lin CH, Wang SC, Ou TT, Li RN, Tsai WC, Liu HW, Yen JH (2008) IkappaBalpha promoter polymorphisms in patients with systemic lupus erythematosus. J Clin Immunol 28(3):207–213
Lisi S, Sisto M (2010) Effects of biological drug adalimumab on tumour necrosis factor-alpha-converting enzyme activation. Immunol Cell Biol 88(3):297–304
Lisi S, Sisto M, Soleti R, Saponaro C, Scagliusi P, D’Amore M, Saccia M, Maffione AB, Mitolo V (2007) Fcgamma receptors mediate internalization of anti-Ro and anti-La autoantibodies from Sjögren’s syndrome and apoptosis in human salivary gland cell line A-253. J Oral Pathol Med 36(9):511–523
Lisi S, D’Amore M, Lofrumento D, Mitolo V, Frassanito MA, Dammacco F, Scagliusi P, Sisto M (2008) Modulation of the Fcγ receptors induced by anti-Ro and anti-La autoantibodies: observations in salivary gland cells. Rheum Int 28(9):943–948
Lisi S, D’Amore M, Scagliusi P, Mitolo V, Sisto M (2009) Anti-Ro/SSA autoantibody-mediated regulation of extracellular matrix fibulins in human epithelial cells of the salivary gland. Scand J Rheumatol 38(3):198–206
Lisi S, Sisto M, Lofrumento DD, Cucci L, Frassanito MA, Mitolo V, D’Amore M (2010a) Pro-inflammatory role of Anti-Ro/SSA autoantibodies through the activation of Furin-TACE-amphiregulin axis. J Autoimmun 35(2):160–170
Lisi S, Sisto M, Lofrumento DD, Frassanito MA, Caprio S, Romano ML, Mitolo V, D’Amore M (2010b) Regulation of mRNA caspase-8 levels by anti-nuclear autoantibodies. Clin Exp Med 10(3):199–203
Lisi S, Sisto M, Lofrumento DD, D’Amore S, D’Amore M (2011) Advances in the understanding of the Fc gamma receptors-mediated autoantibodies uptake. Clin Exp Med 11(1):1–10
Lisi S, Sisto M, Lofrumento DD, D’Amore M (2012a) Sjögren’s syndrome autoantibodies provoke changes in gene expression profiles of inflammatory cytokines triggering a pathway involving TACE/NF-κB. Lab Invest 92:615–624
Lisi S, Sisto M, Lofrumento DD, D’Amore M (2012b) Altered IκBα expression promotes NF-κB activation in monocytes from primary Sjögren’s syndrome patients. Pathology 44(6):557–561
Lisi S, Sisto M, Lofrumento DD, D’Amore M, De Lucro R, Ribatti D (2012b) A potential role of the GRO-α/CXCR2 system in Sjögren’s syndrome: regulatory effects of pro-inflammatory cytokines. Histochem Cell Biol (in press)
Manoussakis MN, Kapsogeorgou EK (2007) The role of epithelial cells in the pathogenesis of Sjögren’s syndrome. Clin Rev Allergy Immunol 32:225–230
Miterski B, Bohringer S, Klein W, Sindern E, Haupts M, Schimrigk S, Epplen JT (2002) Inhibitors in the NFkappaB cascade comprise prime candidate genes predisposing to multiple sclerosis, especially in selected combinations. Genes Immun 3(4):211–219
Moutsopoulos HM (1994) Sjögren’s syndrome: autoimmune epithelitis. Clin Immunol Immunopathol 72:162–165
Nguyen CQ, Sharma A, She JX, McIndoe RA, Peck AB (2009) Differential gene expressions in the lacrimal gland during development and onset of keratoconjunctivitis sicca in Sjögren’s syndrome (SJS)-like disease of the C57BL/6.NOD-Aec1Aec2 mouse. Exp Eye Res 88:398–409
Ou TT, Lin CH, Lin YC, Li RN, Tsai WC, Liu HW, Yen JH (2008) IkappaBalpha promoter polymorphisms in patients with primary Sjögren’s syndrome. J Clin Immunol 28(5):440–444
Peng B, Ling J, Lee AJ, Wang Z, Chang Z, Jin W, Kang Y, Zhang R, Shim D, Wang H, Fleming JB, Zheng H, Sun SC, Chiao PJ (2010) Defective feedback regulation of NF-kappaB underlies Sjogren’s syndrome in mice with mutated kappaB enhancers of the IkappaBalpha promoter. Proc Natl Acad Sci USA 107(34):15193–15198
Schneider C, Strayhorn WD, Brantley DM, Nanney LB, Yull FE, Brash AR (2004) Upregulation of 8-lipoxygenase in the dermatitis of IkappaB-alpha-deficient mice. J Invest Dermatol 122:691–698
Sens DA, Hintz DS, Rudisill MT, Sens MA, Spicer SS (1985) Explant culture of human submandibular gland epithelial cells: evidence for ductal origin. Lab Invest 52(5):559–567
Sisto M, Lisi S, Castellana D, Scagliusi P, D’Amore M, Caprio S, Scagliusi A, Acquafredda A, Panaro MA, Mitolo V (2006) Autoantibodies from Sjogren’s syndrome induce activation of both the intrinsic and extrinsic apoptotic pathways in human salivary gland cell line A-253. J Autoimmun 27(1):38–49
Sisto M, Lisi S, Lofrumento DD, D’Amore M, Scagliusi P, Mitolo V (2007) Autoantibodies from Sjögren’s syndrome trigger apoptosis in salivary gland cell line. Ann N Y Acad Sci 1108:418–425
Sisto M, D’Amore M, Scagliusi P, Mitolo V, Lisi S (2008) Selective TNF-alpha gene silencing attenuates apoptosis in human salivary gland epithelial cells. Int J Immunopathol Pharmacol 2(4):1045–1047
Sisto M, D’Amore M, Caprio S, Mitolo V, Scagliusi P, Lisi S (2009a) Tumor necrosis factor inhibitors block apoptosis of human epithelial cells of the salivary glands. Ann N Y Acad Sci 1171:407–414
Sisto M, D’Amore M, Lofrumento DD, Scagliusi P, D’Amore S, Mitolo V, Lisi S (2009b) Fibulin-6 expression and anoikis in human salivary gland epithelial cells: implication in Sjogren’s syndrome. Int Immunol 21:303–311
Sisto M, Lisi S, Lofrumento DD, Frassanito MA, Cucci L, D’Amore S, Mitolo V, D’Amore M (2009c) Induction of TNF-alpha-converting enzyme-ectodomain shedding by pathogenic autoantibodies. Int Immunol 21(12):1341–1349
Sisto M, Lisi S, Lofrumento DD, Caprio S, Mitolo V, D’Amore M (2010a) TNF blocker drugs modulate human TNF-alpha-converting enzyme pro-domain shedding induced by autoantibodies. Immunobiology 215(11):874–883
Sisto M, Lisi S, Lofrumento DD, Ingravallo G, Mitolo V, D’Amore M (2010b) Expression of pro-inflammatory TACE-TNF-α-amphiregulin axis in Sjögren’s syndrome salivary glands. Histochem Cell Biol 134(4):345–353
Sisto M, Lisi S, Lofrumento DD, Ingravallo G, Maiorano E, D’Amore M (2011) A failure of TNFAIP3 negative regulation maintains sustained NF-κB activation in Sjögren’s syndrome. Histochem Cell Biol 135(6):615–625
Sisto M, Lisi S, Lofrumento DD, D’Amore M, Frassanito MA, Ribatti D (2012a) Sjögren’s syndrome pathological neovascularization is regulated by VEGF-A-stimulated TACE-dependent crosstalk between VEGFR2 and NF-κB. Genes Immun 13(5):411–420
Sisto M, Lisi S, Lofrumento DD, D’Amore M, Ribatti D (2012b) Neuropilin-1 is upregulated in Sjögren’s syndrome and contributes to pathological neovascularization. Histochem Cell Biol 137:669–677
Tak PP, Firestein GS (2001) NF-kappaB: a key role in inflammatory diseases. J Clin Invest 107:7–11
Tas SW, Vervoordeldonk MJ, Hajji N, May MJ, Ghosh S, Tak PP (2006) Local treatment with the selective IkappaB kinase beta inhibitor NEMO-binding domain peptide ameliorates synovial inflammation. Arthritis Res Ther 8:R86
Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, Pillemer SR, Talal N, Weisman MH (2002) Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American–European consensus group. European study group on classification criteria for Sjögren’s syndrome. Ann Rheum Dis 61:554–558
Voulgarelis M, Tzioufas AG (2010) Pathogenetic mechanisms in the initiation and perpetuation of Sjögren’s syndrome. Nat Rev Rheumatol 6:529–537
Yamamoto Y, Gaynor RB (2001) Role of the NF-kappaB pathway in the pathogenesis of human disease states. Curr Mol Med 1(3):287–296
Acknowledgments
We are grateful to M.V.C. Pragnell, B.A., for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Margherita Sisto and Sabrina Lisi have equal contribution in this work and both are equally considered as “first author”.
Rights and permissions
About this article
Cite this article
Sisto, M., Lisi, S., Lofrumento, D.D. et al. Salivary gland expression level of IκBα regulatory protein in Sjögren’s syndrome. J Mol Hist 44, 447–454 (2013). https://doi.org/10.1007/s10735-013-9487-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-013-9487-6