Abstract
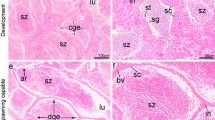
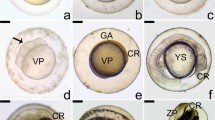
Extracellular matrix in the ovarian follicle has been characterised for several mammalian species but there are no reports that describe the immunolocalisation of the extracellular matrix elements, matrix metalloproteinases, and its relation to plasma 17β estradiol levels and follicular apoptosis during the teleost’s reproductive cycle. The present study used immunohistochemistry to characterise the distribution of laminin β2, collagen type IV, fibronectin and matrix metalloproteinases-9 (MMP-9). The TUNEL in situ technique was used to quantify apoptosis and indirect immunofluorimetric to determine plasma 17β estradiol levels. The TUNEL-positive reaction associated with morphological features exhibited follicular apoptosis. During postovulatory follicle involution, the drop in plasma 17β estradiol levels after spawning contributed to the intense apoptosis observed. By immunohistochemical analysis, laminin β2 and collagen type IV were identified as the major constituents of the basement membrane. The loss of integrity of the basement membrane occurred due to lyses of the major constituents, and coincides with increased follicular apoptosis. The integrity of the basement membrane is important for the survival of follicular cells. Furthermore, the MMP-9 results suggest that this enzyme is involved in final oocyte maturation and regression of postovulatory follicles. Fibronectin was observed on the surface of follicular cells of the postovulatory follicle in P. argenteus, this being important for maintaining normal cell adhesion to extracellular matrix. In conclusion, our results suggest that the structure and composition of the extracellular matrix, and plasma 17β estradiol levels related to apoptosis, play an important role during the follicular development and post-spawning involution in teleost fishes.








Similar content being viewed by others
References
Berkholtz CB, Lai BE, Woodruff TK, Shea LD (2006a) Distribution of extracellular matrix proteins type I collagen, type IV collagen, fibronectin, and laminin in mouse folliculogenesis. Histochem Cell Biol 126:583–592
Berkholtz CB, Shea LD, Woodruff TK (2006b) Extracellular matrix functions in follicle maturation. Semin Reprod Med 24:262–269
Boudreau N, Werb Z, Bissel MJ (1996) Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc Natl Acad Sci 93:3509–3513
Câmara-Pereira ES, Campos LM, Vanier-Santos MA, Mermelstein CS, Costa ML (2009) Distribution of cytoskeletal and adhesion proteins in adult zebrafish skeletal muscle. Hist Histopathol 24:187–196
Carnegie JA (1990) Secretion of fibronectin by rat granulosa cells occurs primarily during early follicular development. J Reprod Fertil 89:579–589
Chun SY, Eisenhauer KM, Minami S, Billig H, Perlas E, Hsueh AJW (1996) Hormonal regulation of apoptosis in early antral follicles: follicle-stimulating hormone as a major survival factor. Endocrinol 137:1447–1456
Curry TE, Osteen KG (2001) Cyclic changes in the matrix metalloproteinase system in the ovary and uterus. Biol Reprod 64:1285–1296
Curry TE, Osteen KG (2003) The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev 24:428–465
Drevinick PE, Sandheinrich MB, Oris JT (2006) Increased ovarian follicular apoptosis in fathead minnows (Pimephales promelas) exposed to dietary methylmercury. Aquat Toxicol 79:49–54
Drummond CD, Bazzoli N, Rizzo E, Sato Y (2000) Postovulatory follicle: a model for experimental studies of programmed cell death or apoptosis in teleost. J Exp Zool 287:176–182
Frisch SM, Francis H (1994) Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol 124:619–626
Grier HJ, Uribe MC, Parenti LR (2007) Germinal epithelium, folliculogenesis, and postovulatory follicles in ovaries of rainbow trout, Oncorhynchus mykiss (Walbaum, 1972) (Teleostei, Protacanthopterygii, Salmoniformes). J Morphol 268:293–310
Guthrie HD, Cooper BS, Welch GR, Zakaria AD, Johnson LA (1995) Atresia in follicles grown after ovulation in the pig: measurement of increased apoptosis in granulosa cells and reduced follicular fluid 17β estradiol. Biol Reprod 52:920–927
Hensey C, Gauter J (1998) Programmed cell death during Xenopus development: a spatial-temporal analysis. Develop Biol 203:36–48
Hulboy DL, Rudolph LA, Matrisian LM (1997) Matrix metalloproteinases as mediators of reproductive function. Mol Hum Reprod 3:27–45
Irving-Rodgers HF, Rodgers RJ (2005) Extracellular matrix in ovarian follicular development and disease. Cell Tissue Res 322:89–98
Ito T, Kobayashi Y, Morita T, Horimoto T, Kawaoka Y (2002) Virulent influenza A viruses induce apoptosis in chickens. Virus Res 84:27–35
Iwahashi M, Muragaki Y, Ooshima A, Nakano R (2000) Type VI collagen expression during growth of human ovarian follicles. Fert Steril 74:343–347
Jalabert B (2005) Particularities of reproduction and oogenesis in teleost fish compared to mammals. Reprod Nutr Dev 45:261–279
Janz DM, Van Der Kraak G (1997) Supression of apoptosis by gonadotropin 17β estradiol and epidermal growth factor in rainbow trout preovulatory ovarian follicles. Gen Comp Endocrinol 105:186–193
Kaptaner B, Ural G (2006) Apoptosis in postovulatory follicles of Chalcalburnus tarichi Pallas, 1811. J Fish Aquat Sci 23:263–267
Kerr JFR, Willie AH, Currie AR (1972) Apoptosis: basis biological phenomenon with wide ranging implicatons in tissue kinetics. Br J Cancer 26:239–257
Kook S, Kim DH, Shim SR, Kim H, Chun J-S, Song WK (2003) Caspase-dependent cleavage of tensin induces disruption of actin cytoskeleton during apoptosis. Biochem Biophys Res Commun 303:37–45
Korpos E, Wu C, Song J, Hallmann R, Sorokin L (2009) Role of the extracellular matrix lymphocite migration. Cell Tissue Res. doi: 10.1007/s0441-009-0853-3
Korsnes MS, Hetland DL, Espenes A, Aune T (2007) Cleavage of tensin during cytoskeleton disruption in YTX-induced apoptosis. Toxicol In Vitro 21:9–15
Kulms D, DuBmann H, Poppelmann B, Stander S, Schwarz A, Schwarz T (2002) Apoptosis induced by disruption of the actin cytoskeleton is mediated via activation of CD95 (Fas/APO-1). Cell Death Differ 9:598–608
Lang DM, Romero-Aleman MM, Arbelo-Galvan J, Sturmer AO, Monzon-Mayor M (2002) Regeneration of retinal axon in the lizard Gallotia galloti is not linked to regeneration of new retinal ganglions cells. J Neurobiol 52:322–335
Le Bellego F, Pisselet C, Huet C, Monget P, Monniaux D (2002) Laminin-alpha6beta1 integrin interaction enhances survival and proliferation and modulates steroidogenesis of ovine granulosa cells. J Endocrinol 172:45–59
Matsui H, Ogiwara K, Ohkura R, Yamashita M, Takahashi T (2000) Expression of gelatinases A and B in the ovary of the medaka fish Oryzias latipes. Eur J Biochem 267:4658–4667
Narkar M, Kholkute S, Nandedkar T (2006) Hormonal regulation of apoptosis in the endometrium of common marmosets (Callithrix jacchus). Theriogenology 66:1194–1209
Ndozangue-Touriguine O, Hamelin J, Breàrd J (2008) Cytoskeleton and apoptosis. Biochem Pharmacol 76:11–18
Ogiwara K, Takano N, Shinohara M, Murakami M, Takahashi T (2005) Gelatinase A and membrane-type matrix metalloproteinases 1 and 2 are responsible for follicle rupture during ovulation in the medaka. Proc Natl Acad Sci 102:8442–8447
Ortiz-Delgado JB, Sarasquete C (2004) Toxicity, histopathological alterations and immunohistochemical CYP1A induction in the early life stages of the seabream, Sparus aurata, following waterborne exposure to B(a)P and TCDD. J Mol Hist 35:29–45
Quagio-Grassiotto I, Guimarães ACD (2006) Follicular ephitellium, theca and egg envelope formation in Serrasalmus spilopleura (Teleostei, Characiformes, Characidae). Acta Zool 84:121–129
Rodgers RJ, Irving-Rodgers HF, Russell DL (2003) Extracellular matrix of the developing ovarian follicle. Reproduction 126:415–424
Romagosa E, Godinho HM, Nahara MY (1985) Tipo de desova do curimbatá Prochilodus scrofa do Rio Mogi-Guaçu, Pirassununga, SP. Rev Bras Reprod Ani 8:113–120
Santos HB, Rizzo E, Bazzoli N, Sato Y, Moro L (2005) Ovarian regression and apoptosis in the South American teleost Leporinus taeniatus Lütken (Characiformes, Anostomidae) from the São Francisco Basin. J Fish Biol 67:1446–1459
Santos JE, Padilha GEV, Bomcompagni-Júnior O, Santos GB, Rizzo E, Bazzoli N (2006) Ovarian follicle growth in the catfish Iheringichthys labrosus (Siluriformes: Pimelodidae). Tissue Cell 38:303–310
Santos HB, Sato Y, Moro L, Bazzoli N, Rizzo E (2009) Relationship among follicular apoptosis, integrin β1 and collagen type IV during early ovarian regression in the teleost Prochilodus argenteus after induced spawning. Cell Tissue Res 332:159–170
Sato Y, Cardoso EL, Godinho AL, Godinho HP (1996) Hypofysation parameters of the fish Prochilodus marggravii obtained in routine hatchery station conditions. Rev Bras Biol 56:59–64
Senthilkumaran B, Yoshikuni M, Nagahama Y (2004) A shift in steroidogenesis occuring in ovarian follicles prior to oocyte maturation. Mol Cell Endocrinol 215:11–18
Sharma D, Kinsey WH (2006) Fertilization triggers localized activation of Src-family protein kinases in the zebrafish egg. Develop Biol 295:604–614
Smith MF, Ricke WA, Bakke LJ, Dow MPD, Smith GW (2002) Ovarian tissue remodeling: role of matrix metalloproteinases and their inhibitors. Mol Cell Endocrinol 191:45–56
Sternilicht MD, Werb Z (2001) How matrix metalloproteinase regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516
Suzukawa M, Komiya A, Iikura M, Nagase H, Yoshimura-Uchiyama C, Yamada H, Kawasaki H, Ohta K, Matsushima K, Hirai K, Yamamoto K, Yamaguchi M (2006) Trans-basement membrane migration of human basophils: role of matrix metalloproteinase-9. Int Immunol 18:1575–1583
Svoltys M, Tabarowski Z, Pawlik A (2000) Apoptosis of postovulatory cumulus granulosa cells of the rat. Anat Embryol 202:523–529
Takle H, Andersen O (2007) Caspases and apoptosis in fish. J Fish Biol 71:326–349
Thomé RG, Santos HB, Arantes FP, Prado PS, Domingos FFT, Sato Y, Bazzoli N, Rizzo E (2006) Regression of post-ovulatory follicles in Prochilodus costatus Valenciennes, 1850 (Characiformes, Prochilodontidae). Braz J Morphol Sci 23:495–500
Thomé RG, Santos HB, Arantes FP, Domingos FFT, Bazzoli N, Rizzo E (2009) Dual roles for autophagy during follicular atresia in fish ovary. Autophagy 5:1–3
Van Wezel IL, Rodgers HF, Rodgers RJ (1998) Differencial localization of laminin chains in the bovine follicle. J Reprod Fertil 112:1334–1341
Zhao Y, Luck MR (1995) Gene expression and protein distribution of collagen, fibronectin and laminin in bovine follicles and corpora lutea. J Reprod Fertil 104:115–123
Acknowledgment
This work was supported by CNPq, FAPEMIG, CAPES and CODEVASF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thomé, R., dos Santos, H.B., Sato, Y. et al. Distribution of laminin β2, collagen type IV, fibronectin and MMP-9 in ovaries of the teleost fish. J Mol Hist 41, 215–224 (2010). https://doi.org/10.1007/s10735-010-9281-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-010-9281-7