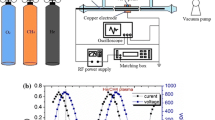
Abstract
The kinetics of methane pyrolysis stimulated by the introduction of atomic hydrogen into the reaction medium from an arcjet plasma source was analyzed. Numerical simulation of the reaction kinetics demonstrated that the thermal pyrolysis at lower temperatures (1800 K or lower) followed the radical chain mechanism with short chains (a chain length of 2 or 3), and the addition of atomic hydrogen considerably increased the rate of the process. An analysis of the kinetics of pyrolysis in a stirred reactor showed that acetylene was formed immediately after methane degradation without the buildup of by-products in the reaction medium.
Similar content being viewed by others
REFERENCES
Baranov, I.E., Demkin, S.A., Zhivotov, V.K., et al., Khim. Vys. Energ., 2004, vol. 38, no.3, p. 222 [High Energy Chem., 2004, vol. 38, no. 3, p. 191].
Knorre, E., Kurs khimicheskoi kinetiki (Chemical Kinetics Textbook), Moscow: Nauka, 1973.
Kassel, L.S., J. Am. Chem. Soc., 1932, vol. 54, p. 3949.
Frenclach M., J. Colloid Interface Sci., 1987, vol. 118, p. 485.
NIST Database, US Department of Commerce, National American Institute of Standards and Technology, 1994.
“Workbench.” Programma firmy “Kintekh” (The Workbench Software Developed by Kintekh), Russia.
Khimicheskaya entsiklopediya (Chemical Encyclopedia), Knunyants, I.L., Ed., Moscow: Bol’shaya Rossiiskaya Entsiklopediya, 1992, vol. 3, p. 535.
Neftekhimicheskaya promyshlennost’. Spravochnik (Petrochemical Industry: A Handbook), Moscow: Neft’ i gaz, 1999.
Polak, L.S., Ovsyannikov, A.A., and Slovetskii, D.I., Teoreticheskaya i prikladnaya plazmokhimiya (Theoretical and Applied Plasma Chemistry), Moscow: Nauka, 1975.
Zhorov, Yu.M., Kinetika promyshlennykh organicheskikh reaktsii (Kinetics of Industrial Organic Reactions), Moscow: Khimiya, 1989.
Zhorov, Yu.M., Termodinamika i kinetika reaktsii v uglevodorodakh (Thermodynamics and Kinetics of Reactions in Hydrocarbons), Moscow: Nauka, 1987.
Ovsyannikov, V.A., Turbulentnye strui v plazme (Turbulent Jets in Plasma), Moscow: Nauka, 1985.
Author information
Authors and Affiliations
Additional information
__________
Translated from Khimiya Vysokikh Energii, Vol. 39, No. 4, 2005, pp. 312–316.
Original Russian Text Copyright © 2005 by Baranov, Demkin, Zhivotov, Nikolaev, Rusanov, Fedotov.
Rights and permissions
About this article
Cite this article
Baranov, I.E., Demkin, S.A., Zhivotov, V.K. et al. Methane Pyrolysis Stimulated by Admixture of Atomic Hydrogen: 2. Mechanism Analysis and Kinetics Calculation. High Energy Chem 39, 268–272 (2005). https://doi.org/10.1007/s10733-005-0053-y
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10733-005-0053-y