Abstract
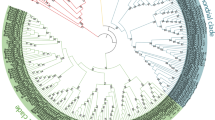
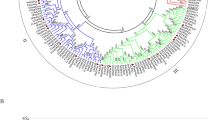
Type 2C protein phosphatase (PP2C) is an important component of protein phosphatases involved in various biological processes, such as plant growth and development, cell cycle regulation, signal transduction, and environmental stress response. ABI1 (ABA insensitive 1), a member of the PP2C gene family, is a negative regulator in abscisic acid (ABA) signal transduction possibly related to stress tolerance, but ABI1 in economical plants is poorly understood. In this study, BcABI1 was isolated from Chinese cabbage [Brassica campestris (syn. B. rapa) L. ssp. chinensis (L) Makino] and 23 other cruciferous plants. Phylogenetic analysis was conducted according to the gene sequence of AtABI1 in Arabidopsis. Three introns are present in ABI1 genes encoding 400–437 amino acids. Multiple alignment of the sequences of 24 ABI1 genes showed that the ABI1 homologous genes of cruciferous plants were highly conserved. Meanwhile, the Ka/Ks values were obtained from orthologous gene pairs between the At genome and other crucifer crop genomes, and results showed that the natural selective pressures on these genes were purifying selection. Subsequently, the structure and expression profiles of BcABI1 were examined under abiotic and biotic stresses. BcABI1 presented a constitutive expression pattern, participating in wound stress and NaCl stress responses, and also induced by biotic stress in Chinese cabbage inoculated with Botrytis cinerea and Sclerotinia sclerotiorum. A significant difference between the pathogenic ability of the two bacteria can be observed, and the S. sclerotiorum caused faster pathogenesis. These results could help to elucidate the function of BcABI1 in plant growth and stress response, and gain valuable insights into Chinese cabbage breeding for the improvement of multiple-stress-tolerance.




Similar content being viewed by others
References
Babula-Skowronska D, Ludwikow A, Ciesla A, Olejnik A, Cegielska-Taras T, Bartkowiak-Broda I, Sadowski J (2015) Involvement of genes encoding ABI1 protein phosphatases in the response of Brassica napus L. to drought stress. Plant Mol Biol 88(4–5):445–457
Banerjee A, Roychoudhury A (2017) Abscisic-acid-dependent basic leucine zipper (bZIP) transcription factors in plant abiotic stress. Protoplasma 254:3–16
Boratko A, Csortos C (2017) TIMAP, the versatile protein phosphatase 1 regulator in endothelial cells. IUBMB Life 69(12):918–928
Bork P, Brown NP, Hegyi H, Schultz J (1996) The protein phosphatase 2C (PP2C) superfamily: detection of bacterial homologues. Protein Sci 5(7):1421–1425
Chen YP, Cao JS, Miao Y, Ye WZ (2000) Analysis of genetic polymorphisms in vegetable crops of Brassica campestris by RAPD markers. J Zhejiang Univ (Agri Life Sci) 26(2):131–136
Chen HY, Hsieh EJ, Cheng MC, Chen CY, Hwang SY, Lin TP (2016) ORA47 (octadecanoid-responsive AP2/ERF-domain transcription factor 47) regulates jasmonic acid and abscisic acid biosynthesis and signaling through binding to a novel cis-element. New Phytol 211(2):599–613
Clouse SD (2016) Brassinosteroid/Abscisic acid antagonism in balancing growth and stress. Dev Cell 38(2):118–120
Cristianini N, Hahn MW (2007) Introduction to computational genomics: a case studies approach. Cambridge, Cambridge University Press
Darvishi E, Kahrizi D, Ghaheri M, Yari K (2017) Study on highly conserved motifs of PP2C-type protein phosphatases in some plant species. Biharean Biologist 11(2):130–132
Ding Z, Li S, An X, Liu X, Qin H, Wang D (2009) Transgenic expression of MYB15 confers enhanced sensitivity to abscisic acid and improved drought tolerance in Arabidopsis thaliana. J Genet Genomics 36(1):17–29
Fuchs S, Grill E, Meskiene I, Schweighofer A (2013) Type 2C protein phosphatases in plants. FEBS J 280(2):681–693
Fujita Y, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K (2011) ABA-mediated transcriptional regulation in response to osmotic stress in plants. J Plant Res 124:509–525
Gao S, Song JB, Wang Y, Yang ZM (2017) An F-box E3 ubiquitin ligase-coding gene AtDIF1 is involved in Arabidopsis salt and drought stress responses in an abscisic acid-dependent manner. Environ Exp Bot 138:21–35
Heyman J, Canher B, Bisht A, Christiaens F, De Veylder L (2018) Emerging role of the plant ERF transcription factors in coordinating wound defense responses and repair. J Cell Sci 131(2):208215
Kim YY, Cui MH, Noh MS, Jung KW, Shin JS (2017) The FBA motif-containing protein AFBA1 acts as a novel positive regulator of ABA response in Arabidopsis. Plant Cell Physiol 58(3):574–586
Komatsu K, Nishikawa Y, Ohtsuka T, Taji T, Quatrano RS, Tanaka S, Sakata Y (2009) Functional analyses of the ABI1-related protein phosphatase type 2C reveal evolutionarily conserved regulation of abscisic acid signaling between Arabidopsis and the moss Physcomitrella patens. Plant Mol Biol 70:327–340
Kwon T (2016) Cytokinin response factor 2 positively regulates salicylic acid-mediated plant immunity in Arabidopsis thaliana. Plant Biotechnol 33(3):207–210
Lefoulon C, Boeglin M, Moreau B, Very AA, Szponarski W, Dauzat M, Michard E, Gaillard I, Cherel I (2016) The Arabidopsis AtPP2CA protein phosphatase inhibits the GORK K+ efflux channel and exerts a dominant suppressive effect on phosphomimetic-activating mutations. J Biol Chem 291(12):6521–6533
Li Y, Li Y, Liu Y, Wu Y, Xie Q (2018) The sHSP22 heat shock protein requires the ABI1 protein phosphatase to modulate polar auxin transport and downstream responses. Plant Physiol 176(3):2406–2425
Liu ZN, Yu XL, Wang FZ, Hu S, Liu YP, Lu G (2012) Physiological, biochemical, and molecular characterization of a new female sterile mutant in turnip. Plant Growth Regul 68:239–248
Liu SY, Liu YM, Yang XH, Tong CB, Edwards D, Parkin IAP, Zhao MX, Ma JX, Yu JY, Huang SM et al. (2014) The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat Commun 5:3930
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–∆∆CT method. Methods 25(4):402–408
Ludwikow A (2015) Targeting proteins for proteasomal degradation-a new function of Arabidopsis ABI1 protein phosphatase 2C. Front Plant Sci 6:310
Ludwikow A, Ciesla A, Kasprowicz-Maluski A, Mitula F, Tajdel M, Galganski L, Ziolkowski PA, Kubiak P, Maleck A, Piechalak A et al (2014) Arabidopsis protein phosphatase 2C ABI1 interacts with type I ACC synthases and is involved in the regulation of Ozone-induced ethylene biosynthesis. Mol Plant 7(6):960–976
Maria M, Cristina Y, Miguel G, Jorge L, Juan L, Vicent A, Margarita M, Martin M, Lourdes I, Aurelio G, Pedro R, Armando A (2017) Structure of ligand-bound intermediates of crop ABA receptors highlights PP2C as necessary ABA co-receptor. Mol Plant 10(9):1250–1253
Merlot S, Gosti F, Guerrier D, Vavasseur A, Giraudat J (2001) The ABI1 and ABI2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid signalling pathway. Plant J 25:295–303
Moorhead GBG, De Wever V, Templeton G, Kerk D (2009) Evolution of protein phosphatases in plants and animals. Biochem J 417:401–409
Murcia G, Pontin M, Piccoli P (2018) Role of ABA and Gibberellin A3 on gene expression pattern of sugar transporters and invertases in Vitis vinifera cv. Malbec during berry ripening. Plant Growth Regul 84(2):275–283
Nagaharu U (1935) Genome analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Jpn J Bot 7:389–452
Qu YN, Song P, Hu YW, Jin X, Jia QR, Zhang XD, Chen L, Zhang Q (2018) Regulation of stomatal movement by cortical microtubule organization in response to darkness and ABA signaling in Arabidopsis. Plant Growth Regul 84(3):467–479
Rodrigues A, Adamo M, Crozet P, Margalha L, Confraria A, Martinho C, Elias A, Rabissi A, Lumbreras V, Gonzalez-Guzman M et al (2013) ABI1 and PP2CA phosphatases are negative regulators of Snf1-related protein kinase1 signaling in Arabidopsis. Plant Cell 25(10):3871–3884
Schweighofer A, Hirt H, Meskiene I (2004) Plant PP2C phosphatases: emerging functions in stress signaling. Trends Plant Sci 9:236–243
The Brassica rapa Genome Sequencing Project Consortium (2011) The genome of the mesopolyploid crop species Brassica rapa. Nat Genet 43:1035–1039
Ton J, Flors V, Mauch-Mani B (2009) The multifaceted role of ABA in disease resistance. Trends Plant Sci 14(6):310–317
Tougane K, Komatsu K, Bhyan SB, Sakata Y, Ishizaki K, Yamato KT, Kohchi T, Takezawa D (2010) Evolutionarily conserved regulatory mechanisms of abscisic acid signaling in land plants: characterization of ABSCISIC ACID INSENSITIVE 1-like type 2C protein phosphatase in the liverwort Marchantia polymorpha. Plant Physiol 152:1529–1543
Wang HJ, Tang J, Liu J, Hu J, Liu JJ, Chen YX, Cai ZY, Wang XL (2018) Abscisic acid signaling inhibits brassinosteroid signaling through dampening the dephosphorylation of BIN2 by ABI1 and ABI2. Mol Plant 11(2):315–325
Xiang YL, Sun XP, Gao S, Qin F, Dai MQ (2017) Deletion of an endoplasmic reticulum stress response element in a ZmPP2C-A gene facilitates drought tolerance of Maize seedlings. Mol Plant 10(3):456–469
Xue T, Wang D, Zhang S, Ehlting J, Ni F, Jakab S (2008) Genome-wide and expression analysis of protein phosphatase 2C in rice and Arabidopsis. BMC Genomics 9(1):550–570
Yang JH, Liu DY, Wang XW, Ji CM, Cheng F, Liu BN, Hu ZY, Chen S, Pental D, Ju YH, Li XM, Zhang JH, Wang JL, Liu F, Ma WW, Shopan J, Zheng HK, Mackenzie SA, Zhang MF (2016) The genome sequence of allopolyploid Brassica juncea and analysis of differential homoeolog gene expression influencing selection. Nat Genet 48:1225–1232
Yoshida R, Umezawa T, Mizoguchi T, Takahashi S, Takahashi F, Shinozaki K (2006a) The regulatory domain of SRK2E/OST1/SnRK2.6 interacts with ABI1 and integrates abscisic acid (ABA) and osmotic stress signals controlling stomatal closure in Arabidopsis. J Biol Chem 281(8):5310–5318
Yoshida T, Nishimura N, Kitahata N, Kuromori T, Ito T, Asami T, Shinozaki K, Hirayama T (2006b) ABA-Hypersensitive Germination3 encodes a protein phosphatase 2C (AtPP2CA) that strongly regulates abscisic acid signaling during germination among Arabidopsis protein phosphatase 2Cs. Plant Physiol 140:115–126
Yoshida T, Fujita Y, Sayama H, Kidokoro S, Maruyama K, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2010) AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J 61:672–685
Yoshida T, Mogami J, Yamaguchi-shinozaki K (2015) Omics approaches toward defining the comprehensive abscisic acid signaling network in plants. Plant Cell Physiol 56:1043–1052
Yu CL, Zhan YH, Feng XP, Huang ZA, Sun CD (2017) Identification and expression profiling of the auxin response factors in Capsicum annuum L. under abiotic stress and hormone treatments. Int J Mol Sci 18(12):2719
Yuan FF, Wang MY, Hao HM, Zhang YF, Zhao HX, Guo AG, Xu H, Zhou XN, Xie CG (2013) Negative regulation of abscisic acid signaling by the Brassica oleracea ABI1 ortholog. Biochem Biophys Res Commun 442(3–4):202–208
Zhang T, Xiang X, Ye WZ, Yu XL, Cao JS (2006) Molecular cloning and bioinformatic analysis of pollen development related gene BcMF2 from Brassica campestris L. ssp. rapifera. J Zhejiang Univ (Agri Life Sci) 32(6):598–605
Zhang JH, Li XS, He ZM, Zhao XY, Wang QM, Zhou B, Yu DS, Huang XQ, Tang DY, Guo XH, Liu XM (2013) Molecular character of a phosphatase 2C (PP2C) gene relation to stress tolerance in Arabidopsis thaliana. Mol Biol Rep 40(3):2633–2644
Zhao Y, Xing L, Wang XG, Hou YJ, Gao JH, Wang PC, Duan CG, Zhu XH, Zhu JK (2014) The ABA receptor PYL8 promotes lateral root growth by enhancing MYB77-dependent transcription of auxin-responsive genes. Sci Signal 7:328
Zhao Y, Chan ZL, Gao JH, Xing L, Cao MJ, Yu CM, Hu YL, You J, Shi HT, Zhu YF, Gong YH, Mu ZX, Wang HQ, Deng X, Wang PC, Bressan RA, Zhu JK (2016) ABA receptor PYL9 promotes drought resistance and leaf senescence. PNAS 113(7):1949–1954
Acknowledgements
The authors gratefully acknowledge Dr. Gang Lu for his helpful advice and thank Dr. Zhenning Liu, in particular, for the stimulating discussions and critical reading of the manuscript.
Funding
This work was partially supported by the National Key Research and Development Program of China (2016YFD0100204-31), the 948 Project of Agricultural Ministry of China (2014-Z28), National Natural Science Foundation of China (31460521), the Breeding Project of the Sci-tech Foundation of Zhejiang Province (2016C02051-6-1), the Project of the Sci-tech Foundation of Ningbo City(2015C110008), and the Project of Application on Public Welfare Technology in Zhejiang Province(LGN18C150003).
Author information
Authors and Affiliations
Contributions
XY and SH proposed and designed the research. SH, FW and LK performed the experiment under abiotic and biotic stresses. LM, ZK and LK performed the statistical analyses, interpretation of experimental results. CC and DH performed the qRT-PCR analysis. LK and SH wrote the manuscript. LK, HD and XY revised the manuscript.
Corresponding author
Ethics declarations
Disclosures
The authors declare that they have no financial interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10725_2018_399_MOESM1_ESM.tif
Supplemental Fig. 1 Full-length cDNA sequence and deduced amino acid sequence of BcABI1 in Chinese cabbage. (TIF 141338 KB)
10725_2018_399_MOESM8_ESM.tif
Supplemental Fig. 8 Ka/Ks values of BcABI1, BoABI1, BnABI1 and RsABI1 with their orthologous gene AtABI1 over a sliding window of 20 codons. The x-axis indicates the starting codon of sliding window. The y-axis shows the Ka/Ks values. (TIF 47359 KB)
Rights and permissions
About this article
Cite this article
Kong, L., Deng, H., Hu, S. et al. Isolation, expression, and evolution analysis of the type 2C protein phosphatase gene BcABI1 involved in abiotic and biotic stress in Brassica campestris ssp. chinensis. Plant Growth Regul 85, 317–327 (2018). https://doi.org/10.1007/s10725-018-0399-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-018-0399-z