Abstract
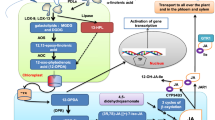
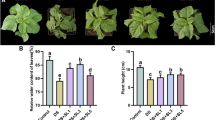
To study the roles of jasmonic acid (JA) and ethylene (ET) pathways in mediating defense against wheat Fusarium head blight (FHB), the expression patterns of genes in the Fusarium graminearum-challenged spikes between Wangshuibai and its susceptible mutant NAUH117 at the Fhb1 locus were compared using wheat microarray. The results showed that most of JA-associated genes were induced in Wangshuibai while only a few were induced in NAUH117, and most ET-associated genes were up-regulated in both genotypes. ELISA assay showed that in the F. graminearum-challenged spikes, endogenous JA content was increased in Wangshuibai while not in NAUH117. Pre-treatment with exogenous methyl JA could decrease the wheat disease severity. However, pretreatment by exogenous ethephon had no such effect. A lipid transfer protein gene, which is a representative gene for JA pathway, was selected for function analysis in Arabidopsis system using a T-DNA insertion mutant line for LTP gene. It was found that the mutant showed compromised FHB resistance compared with its wildtype, proving the possible role of LTP in FHB resistance of Arabidopsis. These results demonstrated that JA pathway should play a critical role in FHB resistance.




Similar content being viewed by others
References
Bai GH, Shaner G (2004) Management and resistance in wheat and barley to Fusarium head blight. Annu Rev Phytopathol 42:135–161
Bari R, Jones J (2009) Role of plant hormones in plant defence responses. Plant Mol Biol 69:473–488
Beckers GJM, Spoel SH (2006) Fine-tuning plant defence signalling: salicylate versus jasmonate. Plant Biol 8:1–10
Buhot N, Gomes E, Milat ML, Ponchet M, Marion D, Lequeu J, Delrot S, Coutos-Thevenot P, Blein JP (2004) Modulation of the biological activity of a tobacco LTP1 by lipid complexation. Mol Biol Cell 15:5047–5052
Chen X, Steed A, Travella S, Keller B, Nicholson P (2009) Fusarium graminearum exploits ethylene signalling to colonize dicotyledonous and monocotyledonous plants. New Phytol 182:975–983
Dong XN (1998) SA, JA, ethylene, and disease resistance in plants. Curr Opin Plant Biol 1:316–323
Fonseca S, Chico JM, Solano R (2009) The jasmonate pathway: the ligand, the receptor and the core signalling module. Curr Opin Plant Biol 12:539–547
Gan Y, Li H, Xie Y, Wu W, Li M, Wang X, Huang J (2014) THF1 mutations lead to increased basal and wound-induced levels of oxylipins that stimulate anthocyanin biosynthesis via COI1 signaling in Arabidopsis. J Integr Plant Biol 56:916–927
Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43:205–227
Golkari S, Gilbert J, Ban T, Procunier JD (2009) QTL-specific microarray gene expression analysis of wheat resistance to Fusarium head blight in Sumai-3 and two susceptible NILs. Genome 52:409–418
Grant M, Lamb C (2006) Systemic immunity. Curr Opin Plant Biol 9:414–420
Hill-Ambroz K, Webb CA, Matthews AR, Li WL, Gill BS, Fellers JP (2006) Expression analysis and physical mapping of a cDNA library of Fusarium head blight infected wheat spikes. Crop Sci 46:S15–S26
Jia HY, Cho SH, Muehlbauer GJ (2009) Transcriptome analysis of a wheat near-isogenic line pair carrying Fusarium head blight-resistant and -susceptible alleles. Mol Plant Microbe Interact 22:1366–1378
Leon-Reyes A, Du YJ, Koornneef A, Proietti S, Korbes AP, Memelink J, Pieterse CMJ, Ritsema T (2010) Ethylene signaling renders the jasmonate response of Arabidopsis insensitive to future suppression by salicylic acid. Mol Plant Microbe Interact 23:187–197
Li GL, Yen Y (2008) Jasmonate and ethylene signaling pathway may mediate Fusarium head blight resistance in wheat. Crop Sci 48:1888–1896
Liechti R, Gfeller A, Farmer EE (2006) Jasmonate signaling pathway. Sci STKE 2006:cm2
Liu SX, Anderson JA (2003) Targeted molecular mapping of a major wheat QTL for Fusarium head blight resistance using wheat ESTs and synteny with rice. Genome 46:817–823
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Loake G, Grant M (2007) Salicylic acid in plant defence: the players and protagonists. Curr Opin Plant Biol 10:466–472
Makandar R, Nalam V, Chaturvedi R, Jeannotte R, Sparks AA, Shah J (2010) Involvement of salicylate and jasmonate signaling pathways in Arabidopsis interaction with Fusarium graminearum. Mol Plant Microbe Interact 23:861–870
Maldonado AM, Doerner P, Dixon RA, Lamb CJ, Cameron RK (2002) A putative lipid transfer protein involved in systemic resistance signalling in Arabidopsis. Nature 419:399–403
Pritsch C, Vance CP, Bushnell WR, Somers DA, Hohn TM, Muehlbauer GJ (2001) Systemic expression of defense response genes in wheat spikes as a response to Fusarium graminearum infection. Physiol Mol Plant Pathol 58:1–12
Schweiger W, Steiner B, Ametz C, Siegwart G, Wiesenberger G, Berthiller F, Lemmens M, Jia HY, Adam G, Muehlbauer GJ, Kreil DP, Buerstmayr H (2013) Transcriptomic characterization of two major Fusarium resistance quantitative trait loci (QTLs), Fhb1 and Qfhs.ifa-5A, identifies novel candidate genes. Mol Plant Pathol 14:772–785
Thatcher LF, Manners JM, Kazan K (2009) Fusarium oxysporum hijacks COI1-mediated jasmonate signaling to promote disease development in Arabidopsis. Plant J 58:927–939
Uppalapati SR, Ishiga Y, Wangdi T, Kunkel BN, Anand A, Mysore KS, Bender CL (2007) The phytotoxin coronatine contributes to pathogen fitness and is required for suppression of salicylic acid accumulation in tomato inoculated with Pseudomonas syringae pv. tomato DC3000. Mol Plant Microbe Interact 20:955–965
Urban M, Daniels S, Mott E, Hammond-Kosack K (2002) Arabidopsis is susceptible to the cereal ear blight fungal pathogens Fusarium graminearum and Fusarium culmorum. Plant J 32:961–973
Van Loon LC, Rep M, Pieterse CMJ (2006) Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol 44:135–162
Van Loon LC, Van Strien EA (1999) The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol 55:85–97
Wang KLC, Li H, Ecker JR (2002) Ethylene biosynthesis and signaling networks. Plant Cell 14:S131–S151
Xiao J, Jia XP, Wang HY, Zhao RH, Fang YH, Gao RH, Wu ZZ, Cao AZ, Wang J, Xue ZK, Zhao WP, Kang JX, Chen QG, Chen PD, Wang XE (2011) A fast-neutron induced chromosome fragment deletion of 3BS in wheat landrace Wangshuibai increased its susceptibility to Fusarium head blight. Chromosome Res 19:225–234
Xiao J, Jin X, Jia X, Wang H, Cao A, Zhao W, Pei H, Xue Z, He L, Chen Q, Wang X (2013) Transcriptome-based discovery of pathways and genes related to resistance against Fusarium head blight in wheat landrace Wangshuibai. BMC Genom 14:197
Acknowledgments
This work was supported by Natural Science Foundation of Jiangsu Province (Grant No. BK20140700, BK20141370), the Fundamental Research Funds for the Central Universities (Grant No. KJ2013003), the National Natural Science Foundation of China (Grant No. 31501305), Jiangsu Science and Technology Support Program (BE2013439), Jiangsu Agricultural Science and Technology Innovation Fund (CX151001), the Program of Introducing Talents of Discipline to Universities (No. B08025), Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and Six Talent Peaks project in Jiangsu Province.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yuxin Sun and Jin Xiao contributed equally to this article.
Rights and permissions
About this article
Cite this article
Sun, Y., Xiao, J., Jia, X. et al. The role of wheat jasmonic acid and ethylene pathways in response to Fusarium graminearum infection. Plant Growth Regul 80, 69–77 (2016). https://doi.org/10.1007/s10725-016-0147-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-016-0147-1