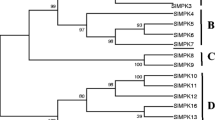
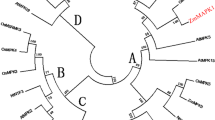
Abstract
The mitogen-activated protein kinase (MAPK) cascades have been previously implicated in signal transduction during plant responses to various environmental stresses. As the convergent point of the MAPK cascades, MAPKKs play paramount roles in amplifying, integrating, and channeling information between the extracellular stimuli and intracellular responses. However, the functional role of MAPKKs in Lycium chinense has never been explored. In this study, a novel MAPKK gene, LcMKK, in L. chinense belonging to group A MAPKKs was isolated and functionally characterized. The transcript level of LcMKK rapidly increased in L. chinense after drought treatments. Overexpression of LcMKK in tobacco conferred dehydration and drought tolerance. Under dehydration and drought conditions, the transgenic tobacco lines exhibited better water status, less accumulation of reactive oxygen species (ROS), higher levels of germination rate and antioxidant enzyme activity than the wild type. In addition, overexpression of LcMKK enhanced the expression of ROS-related and stress-responsive genes under drought conditions. Taken together, these data demonstrate that LcMKK acts as a positive regulator in dehydration/drought stress responses by either regulating ROS homeostasis through the activation of the cellular antioxidant defense system or modulating transcriptional levels of a variety of stress-associated genes.






Similar content being viewed by others
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Agarwal PK, Gupta K, Jha B (2010) Molecular characterization of the Salicornia brachiata SbMAPKK gene and its expression by abiotic stress. Mol Biol Rep 37:981–986
Andreasson E, Ellis B (2010) Convergence and specificity in the Arabidopsis MAPK nexus. Trends Plant Sci 15:106–113
Cai G, Wang G, Wang L, Pan J, Liu Y, Li D (2014) ZmMKK1, a novel group A mitogen-activated protein kinase kinase gene in maize, conferred chilling stress tolerance and was involved in pathogen defense in transgenic tobacco. Plant Sci 214:57–73
Chen J-Q, Meng X-P, Zhang Y, Xia M, Wang X-P (2008) Over-expression of OsDREB genes lead to enhanced drought tolerance in rice. Biotechnol Lett 30:2191–2198
Chen L et al (2012) Genome-wide identification and analysis of MAPK and MAPKK gene families in Brachypodium distachyon. PLoS One 7:e46744
Dong JZ et al (2013) Selenium increases chlorogenic acid, chlorophyll and carotenoids of Lycium chinense leaves. J Sci Food Agric 93:310–315
Foyer CH, Shigeoka S (2011) Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol 155:93–100
Giannopolitis CN, Ries SK (1977) Superoxide dismutases I. Occurrence in higher plants. Plant Physiol 59:309–314
Hadiarto T et al (2006) Activation of Arabidopsis MAPK kinase kinase (AtMEKK1) and induction of AtMEKK1–AtMEK1 pathway by wounding. Planta 223:708–713
Horsch R, Fry J, Hoffmann N, Eichholtz D, Rogers SA, Fraley R (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231
Huang X-S, Liu J-H, Chen X-J (2010) Overexpression of PtrABF gene, a bZIP transcription factor isolated from Poncirus trifoliata, enhances dehydration and drought tolerance in tobacco via scavenging ROS and modulating expression of stress-responsive genes. BMC Plant Biol 10:230
Hundertmark M, Hincha DK (2008) LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genomics 9:118
Kiegerl S et al (2000) SIMKK, a mitogen-activated protein kinase (MAPK) kinase, is a specific activator of the salt stress–induced MAPK, SIMK. Plant Cell 12:2247–2258
Kim HP et al (2002) Zeaxanthin dipalmitate from Lycium chinense fruit reduces experimentally induced hepatic fibrosis in rats. Biol Pharm Bull 25:390–392
Kong X et al (2011a) ZmMKK4, a novel group C mitogen-activated protein kinase kinase in maize (Zea mays), confers salt and cold tolerance in transgenic Arabidopsis. Plant Cell Environ 34:1291–1303
Kong X, Sun L, Zhou Y, Zhang M, Liu Y, Pan J, Li D (2011b) ZmMKK4 regulates osmotic stress through reactive oxygen species scavenging in transgenic tobacco. Plant Cell Rep 30:2097–2104
Kong F, Wang J, Cheng L, Liu S, Wu J, Peng Z, Lu G (2012) Genome-wide analysis of the mitogen-activated protein kinase gene family in Solanum lycopersicum. Gene 499:108–120
Lu W, Chu X, Li Y, Wang C, Guo X (2013) Cotton GhMKK1 Induces the tolerance of salt and drought stress, and mediates defence responses to pathogen infection in transgenic Nicotiana benthamiana. PLoS One 8:e68503
Mikołajczyk M, Awotunde OS, Muszyńska G, Klessig DF, Dobrowolska G (2000) Osmotic stress induces rapid activation of a salicylic acid–induced protein kinase and a homolog of protein kinase ASK1 in tobacco cells. Plant Cell 12:165–178
Miller G, Suzuki N, CIFTCI-YILMAZ S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:453–467
Mizoguchi T, Ichimura K, Irie K, Morris P, Giraudat J, Matsumoto K, Shinozaki K (1998) Identification of a possible MAP kinase cascade in Arabidopsis thaliana based on pairwise yeast two-hybrid analysis and functional complementation tests of yeast mutants. FEBS Lett 437:56–60
Nadarajah K, Sidek HM (2010) The green MAPKS. Asian J Plant Sci 9:1
Nicole M-C, Hamel L-P, Morency M-J, Beaudoin N, Ellis B, Séguin A (2006) MAP-ping genomic organization and organ-specific expression profiles of poplar MAP kinases and MAP kinase kinases. BMC Genomics 7:223
Pitzschke A, Djamei A, Bitton F, Hirt H (2009) A major role of the MEKK1–MKK1/2–MPK4 pathway in ROS signalling. Mol Plant 2:120–137
Rao KP, Richa T, Kumar K, Raghuram B, Sinha AK (2010) In silico analysis reveals 75 members of mitogen-activated protein kinase kinase kinase gene family in rice. DNA Res 17:139–153
Ravikumar G et al (2014) Stress-inducible expression of AtDREB1A transcription factor greatly improves drought stress tolerance in transgenic indica rice. Transgenic Res 23:421–439
Samuel MA, Ellis BE (2002) Double jeopardy both overexpression and suppression of a redox-activated plant mitogen-activated protein kinase render tobacco plants ozone sensitive. Plant Cell 14:2059–2069
Stulemeijer IJ, Stratmann JW, Joosten MH (2007) Tomato mitogen-activated protein kinases LeMPK1, LeMPK2, and LeMPK3 are activated during the Cf-4/Avr4-induced hypersensitive response and have distinct phosphorylation specificities. Plant Physiol 144:1481–1494
Tena G, Asai T, Chiu W-L, Sheen J (2001) Plant mitogen-activated protein kinase signaling cascades. Curr Opin Plant Biol 4:392–400
Umezawa T, Fujita M, Fujita Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Engineering drought tolerance in plants: discovering and tailoring genes to unlock the future. Curr Opin Biotechnol 17:113–122
Wintermans J, De Mots A (1965) Spectrophotometric characteristics of chlorophylls a and b and their phenophytins in ethanol. BBA 109:448–453
Xing Y, Jia W, Zhang J (2008) AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. Plant J 54:440–451
Zhang A, Jiang M, Zhang J, Tan M, Hu X (2006) Mitogen-activated protein kinase is involved in abscisic acid-induced antioxidant defense and acts downstream of reactive oxygen species production in leaves of maize plants. Plant Physiol 141:475–487
Acknowledgments
This work was supported financially by the National Science and Technology Key Project of China on GMO cultivation for new varieties (No. 2014ZX08003-002B) and the National Natural Science Foundation of China (No. 31271419, No. 31271793 and No. 31401391).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, D., Ji, J., Wang, G. et al. LcMKK, a novel group A mitogen-activated protein kinase kinase gene in Lycium chinense, confers dehydration and drought tolerance in transgenic tobacco via scavenging ROS and modulating expression of stress-responsive genes. Plant Growth Regul 76, 269–279 (2015). https://doi.org/10.1007/s10725-014-9998-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-014-9998-5