Abstract
The frequency at which seed accessions are monitored for viability during storage in genebanks (reciprocal of the monitoring interval) must balance timely detection of loss in viability against monitoring cost (seed depletion and staff resource). Up to three decades of genebank seed germination test results of diverse grasses maintained in the International Livestock Research Institute’s medium-term store (circa 8 °C with 5% moisture content) were evaluated in an attempt to derive recommendations on seed accession monitoring intervals. Six different patterns of variation in germination test results during storage were found amongst seed lots by probit analysis within 29 genera: no trend (6 genera); contrasting trends (positive to negative) (3); common slope of loss in viability (11); common slope of increase in ability to germinate (6); common loss in viability (2); common increase in ability to germinate (1). Recommended monitoring intervals were calculated from the fitted survival curves for each of the 13 genera showing uniformity in loss in viability: the medium-term store expectation of 2–10 years’ maintenance of high viability was met in eight genera, whilst four provided greater survival periods. Furthermore, the 13 genera showing either no trend over period of storage or an increase in ability to germinate during storage also exceeded the expectations for survival periods in medium-term stores. Advice is provided on calculating monitoring intervals for different combinations of initial viability with a wide range of potential regeneration standards.
Similar content being viewed by others
Introduction
Seed accessions are only of value in genebanks if they are viable (Hay and Whitehouse 2017). Moreover, high viability is required to avoid the consequences of loss in viability. These consequences include greater difficulty establishing seedlings from the remaining viable seed in reasonable proportion (Khah et al. 1986; Ellis and Dolman 1988), an increased risk of genetic mutation (Abdalla and Roberts 1968, 1969), and losing genes from genetically-heterogeneous accessions (Roos 1988). Hence, the viability of accessions needs to be monitored at suitable intervals (IBPGR 1976; FAO/IPGRI 1994; FAO 2014). Monitoring intervals (period between tests) should be appropriate. They must balance the risk of damage and potential loss of accessions during storage against the direct (e.g. staff time) and indirect costs (e.g. destructive testing of valuable seeds) of monitoring (Hay and Whitehouse 2017).
Genebanks that have been operating for some considerable time are likely to have accumulated considerable information that may help to quantify the changing status of accessions during medium- or long-term storage. An approach based on the seed viability equation (Ellis and Roberts 1980) has been applied recently, successfully in large part, to analyse medium- and long-term records of accession monitoring tests in Fabiaceae from the International Livestock Research Institute’s (ILRI) medium-term store (MTS) and derive evidence-based recommended monitoring intervals (Ellis et al. 2018).
Here we apply that genus-by-genus analytical approach (Ellis et al. 2018) to evaluate the suitability of data compiled over up to 35 years from monitoring the seed lots of accessions of Poaceae in ILRI’s MTS to derive recommended monitoring intervals. We also consider the calculation of monitoring intervals for combinations of different values of initial viability with a wide range of regeneration standards.
Materials and methods
The MTS at ILRI (a nominal storage environment of circa 8 °C with seeds stored hermetically within sealed, laminated-aluminium packets at 5% moisture content) has operated since 1987. Prior to that, seeds were stored in uncontrolled conditions at room temperature. Monitoring test results of accessions of Poaceae until late 2017 were included in our research. Each separate analysis comprised all accessions tested within a genus. In some cases, all the observations were limited to a single species. Each observation comprised a single germination test result for a seed lot after a known period of storage. Responses were compared amongst different seed lots within each genus. Many accessions were represented by multiple seed lots. Each seed lot was a unique identifiable sample, representing seed of the original accession and/or samples provided by later multiplication(s) in different seasons, and sometimes different sites. Every seed lot with two or more observations during storage in the MTS was included in the analyses.
Different germination test procedures, temperature regimes, durations, and dormancy-breaking treatments were applied throughout the (up to) 35-year set of results (within a seed lot and genus). This potential source of error is a consequence of analysing observations not collected for research, but is realistic in terms of application to genebanking as it is practiced. Germination tests comprised 50 seeds per replicate with two replicates typically, but in some tests fewer seeds were available whilst as many as four 50-seed replicates were tested occasionally. In most tests the caryopses were carefully extracted from the glumes directly prior to these tests. This was done to avoid testing empty seeds and to ensure sufficient numbers of caryopses for sound estimates of ability to germinate. Alternating temperature regimes were usually provided, typically 20/30 °C or 20/35 °C but sometimes 15/25 °C (12 h/12 h or 16 h/8 h thermoperiods), with 25 °C if a constant temperature was provided. Test durations varied widely from 4 to 48 days (the former only when full germination occurred rapidly). Pre-chilling at 5–10 °C with or without pre-treatment with 0.2% potassium nitrate were the two most often applied dormancy-breaking treatments, if provided; seeds were sometimes scarified. The criterion of germination was normal seedling development (ISTA 2013).
The results of successive germination tests on samples of seed lots removed from the MTS after different periods of storage were subjected to simultaneous probit analysis, combining the results of all seed lots within a genus, using GENSTAT (Version 18; VSN International Ltd, Hemel Hempstead, UK). These analyses provided estimates of Ki (intercept) and 1/σ (slope) in accordance with
where g is probit percentage ability to germinate after P years in storage for these analyses, and σ is the standard deviation of the frequency distribution of seed deaths in time (Ellis et al. 2018). Equation (1) was modified from the seed viability equation (Ellis and Roberts 1980): negative estimates of 1/σ indicate loss in viability and positive ones greater germination as storage progresses. The two durations p and σ were in years because storage periods were provided in integers of years. Where the database did not identify date of entry into store, the first test result available was assumed as zero time in storage.
A sequence of analyses was followed: estimates of Ki and σ were allowed to vary amongst seed lots within a genus first. Thereafter, each was constrained to a common value in turn, then together. The different constraints were then evaluated (P < 0.05) amongst these models by F tests. This approach of analysing all seed lots together enabled those with only two observations during storage to be included. In some genera, no seed lot provided more than two observations. In these cases no comparisons could be made with the least constrained model (that which allowed both the intercept and slope to vary amongst seed lots). In such cases, differences in slope or intercept were evaluated against a common line for all lots. As before (Ellis et al. 2018), where the common intercept and common slope models were both significant (P = 0.05) and superior to other models, the common slope model was selected. This was because identical values of Ki amongst seed lots are unlikely due to the effects of different production, harvest, and post-harvest environments (Ellis and Roberts 1981). The fitted models were evaluated against observations to test this assumption. Models of common slope (1/σ) with seed lots differing in initial quality (Ki) were compared against all observations analysed within a genus diagrammatically as before for a common storage environment (Ellis and Roberts 1981; Ellis et al. 2018).
Results
The database for Poaceae included 34 genera where the dataset was too small for analysis. In the case of Sporobolus R.Br. as many as 38 observations were available, but this comprised many seed lots with lone observations only at the beginning of storage. The other 33 genera were represented by fewer than 20 observations each, and again most of those individual seed lots were represented by only one germination test result.
This phenomenon was also evident within the 29 genera that were analysed (Table 1). Only 55% of the total number of observations was available for analysis once seed lots represented by a single germination test result (typically on entry into the MTS) were removed.
All six potential outcomes from the analytical approach applied here were detected amongst these 29 genera (Table 1). Six genera provided no significant (P > 0.05) trend for ability to germinate during storage. These genera tended to comprise those with comparatively few observations and/or brief durations of storage in the MTS.
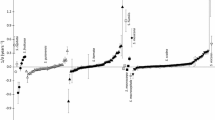
Three genera provided significant trends for ability to germinate during storage with contrasting responses detected amongst seed lots (P < 0.05). In Avena sativa L., these varied between extremes of a loss in viability of 0.14 normal equivalent deviates year−1 (σ = −7.1 years) to an increase in ability to germinate of 0.21 normal equivalent deviates year−1 (σ = 4.8 years) (Fig. 1) amongst the 115 seed lots. Nonetheless, the majority of seed lots provided shallow or no slopes (i.e. little or no change). In Sorghum bicolor Moench, four seed lots provided estimates of rapid loss in viability of − 0.41 to − 0.43 normal equivalent deviates year−1 (σ = −2.3 to −2.4 years) with, at the other extreme, one seed lot providing a positive slope of 0.13 normal equivalent deviates year−1 (σ = 7.7 years) (Fig. 2). The three seed lots of the two remaining Sorghum Moench species were well within this range. Amongst the seven species of Pennisetum Rich., P. pedicellatum Trin. and P. polystachion (L.) Schult. showed the most extreme ranges in estimates of 1/σ amongst seed lots from − 1.79 normal equivalent deviates year−1 (σ = −0.6 years) to 1.46 normal equivalent deviates year−1 (σ = 0.7 years) (Fig. 3).
Variation in slope (1/σ, Eq. 1) amongst 115 seed lots of Avena sativa L. for curves fitted by probit analysis of serial germination test results after different periods in the medium-term store at ILRI. Vertical bars are ± standard errors of the estimates
Variation in slope (1/σ, Eq. 1) amongst 38 seed lots of three species of Sorghum Moench for curves fitted by probit analysis of serial germination test results after different periods in the medium-term store at ILRI. Vertical bars are ± standard errors of the estimates
Variation in slope (1/σ, Eq. 1) amongst 79 seed lots of of seven species of Pennisetum Rich. for curves fitted by probit analysis of serial germination test results after different periods in the medium-term store at ILRI. Vertical bars are ± standard errors of the estimates
Ten genera provided fitted models with a common slope amongst seed lots for loss in viability: i.e. significant loss in viability was detected, with absolute differences in longevity (i.e. Ki varied), but no significant difference in slope (i.e. 1/σ was a common value). An eleventh genus, Paspalum L., was added to this list (Table 1). In this genus, neither 1/σ nor Ki (singly or combined) differed significantly amongst seed lots. Comparison of the observations against fitted models showed the common slope to be superior to the common line model, however. Accordingly the former is reported here (Table 1). Several of the common slope models fitted are compared with the germination test results from monitoring in the MTS in Fig. 4. These examples show the extremes detected in both the rapidity of loss in viability and the variation of observations about the fitted models. Whilst there was a tendency for greater variation about the fitted model to be observed with more observations, there were exceptions. For example, this variation was less in Bothriochloa Kuntze (Fig. 4h) than Phalaris L. (Figure 4g), despite almost two-thirds fewer observations in the latter.
Comparison of observations with generalized seed survival curves, negative cumulative normal distributions fitted by probit analysis, for different seed lots within each of six genera or three species (a. Phleum pratense L. [timothy]; c. Dactylis glomerata L. [cocksfoot, orchard grass]; f. xTriticosecale rimpaui Wittm. [triticale]) in Poaceae stored in the medium-term store at ILRI. Note the different x-axis scales. The fitted seed survival curve within each genus or species is that for the seed lot showing the greatest longevity, i.e. highest estimate of Ki, with the common slope (1/σ, Eq. 1) for all seed lots within that genus. Zero time in storage was offset (i.e. delayed) for the remaining seed lots by the product of the difference in Ki from the best seed lot and the common estimate of σ. The two genera with the most-rapid and the two with the least-rapid loss in viability, for common negative slopes (Table 2), are included here. The parameters of the fitted curves are provided in Table 2
A common line for loss in viability is provided for two genera: Bromus L. and Melinis P. Beauv. (Tables 1 and 2). In both genera the fitted models were, however, poor. In Bromus, a common intercept model was apparently more satisfactory (P = 0.05) than a common slope (P = 0.15) or a common line model (P = 0.27) or indeed independent curves (P = 0.40), but none of the three parsimonious models were an improvement over the latter (P > 0.05). The common line model is presented in Table 2 in order to provide a preliminary estimate of σ for Bromus. In Melinis the common line model (P = 0.004) was the most suitable, but nonetheless provided a poor fit to the observations. Hence in this case also the estimate of σ is preliminary.
Thirteen of the genera investigated provided superior seed longevity to that tabulated for the 13 different genera in Table 2: six showed no significant trend for ability to germinate during storage (P > 0.05), and seven a consistent increase in ability to germinate (P < 0.05) (Table 1). In Brachiaria (Trin.) Griseb. the 88 seed lots did not differ (P > 0.05) in their response of ability to germinate to the period of storage, i.e. the most parsimonious model was appropriate. This common line model (P < 0.05), where Ki = −1.27 (s.e. = 0.0128) and 1/σ = 0.058 (s.e. = 0.002), provided a poor fit to the observations, however. Whilst this model confirmed a significant increase in the ability of Brachiaria seeds to germinate during storage, it was not considered an acceptable model to quantify the observations.
The seed lots within each of the other six genera varied in intercept (Ki) but provided a common slope (1/σ) for an increase in ability to germinate during storage. This was most rapid (least seed-to-seed variability in dormancy release periods) in Eragrostis and slowest (greatest seed-to-seed variability) in Panicum L. (Figure 5). The fitted common slope models described the observations closely in Eragrostis Wolf (Fig. 5a) and Echinochloa P. Beauv. (Figure 5b), whereas in Chloris Sw. (Figure 5c), Setaria P. Beauv. (Figure 5e), and Panicum (Fig. 5f) the observations varied about the fitted lines much more.
Comparison of observations with generalized curves showing increase in ability to germinate, positive cumulative normal distributions fitted by probit analysis, for seed lots within each of five genera or one species (d. Hordeum vulgare L. [barley]) in Poaceae stored in the medium-term store at ILRI. Note the different x-axis scales. The fitted curves shown within each genus or species are for the seed lot providing the lowest estimate of Ki with the common slope (1/σ, Eq. 1) for all seed lots within that genus. Zero time in storage was offset (i.e. delayed) for the remaining seed lots by the product of difference in Ki from the lowest seed lot and the common estimate of σ
Discussion
These analyses of test results in the MTS at ILRI from monitoring accessions of Poaceae, together with previous analyses with Fabaceae (Ellis et al. 2018), have confirmed that advice on monitoring intervals can be derived for a genebank from its own monitoring test records in many, but not all, genera. In proportional terms, the approach was slightly less successful in deriving estimates for monitoring intervals in Poaceae (13 of 63 genera) than previously in Fabaceae (19 of 68 genera), whilst the proportions of genera providing variant estimates of slopes (3 of 63 genera or 5 of 68 genera, respectively)—and so no consistent estimate of monitoring interval could be deduced—were similar and small. The former may be due to the smaller numbers of test results in these genera, and in some cases shorter periods of storage in the MTS. In the current study, the Bromus analyses were somewhat contradictory. Although tabulated here as a common slope amongst seed lots (Tables 1 and 2), a case could be made to also classify the Bromus dataset as either variant slopes or no significant trend.
In both families, the records of monitoring tests for the majority of genera held in the MTS at ILRI did not provide significant evidence of deterioration during storage. This may have been due to insufficient observations, and/or good seed survival over the medium-term, and/or shorter periods of storage in the MTS. Good survival over medium-term seed storage must have been the case in the 7 (Poaceae) and 22 (Fabaceae) genera where significant, consistent, positive trends of ability to germinate with period of storage in the MTS were detected.
Considerable variation was detected amongst the 108 oat (Avena sativa L.) accessions (Fig. 1). Nonetheless, the extreme accession with the poorest longevity survived better over the medium term at 8 °C with 5% moisture content than the 14 oat cultivars stored at room temperature (moisture content not stated) by Aniszewski et al. (2012), where complete loss in viability occurred over 22 years. This comparison illustrates the importance of appropriate environments for successful medium- and long-term seed storage.
Differences in the response of longevity to seed storage environment amongst species have long been quantified (e.g. Ellis and Roberts 1981; Ellis et al. 1982; Ellis and Hong 2007a). Substantial differences in the recommended monitoring interval in the MTS were derived amongst 13 genera in Poaceae (Table 2). This cautions against the use of a single standard monitoring interval for all accessions within a multi-genera gene bank.
As in our earlier study with Fabaceae, we detected substantial improvement in ability to germinate during storage, rather than loss in viability, in many seed lots across several genera of Poaceae. Seed dormancy is considerable in this family, with a wide range of procedures developed to promote the germination of dormant seeds to estimate viability (e.g. Ellis et al. 1985a, b). Dormancy appeared greatest (in the sense that it was lost most slowly during storage) in Chloris, Setaria, and Panicum (Fig. 5c, e, f). All three are especially common in tropical and sub-tropical regions, where strong dormancy aids survival in late seed maturation and subsequently in the soil seed bank. Dormancy also explains the considerable contrast between the increase in ability to germinate during the hermetic storage of seeds of Hordeum vulgare L. detected here (Fig. 5d) and the decline, from 94 to 75% over 23–27 years at 4 °C with about 6% moisture content, reported by van Treuren et al. (2018); and the similar or greater decline, over 32 years’ storage at 0 °C with 6–10% moisture content, reported by Nagel et al. (2009). Avoiding the confounding effects of dormancy in seed viability monitoring tests has long been recognised as a major problem for seed genebanks (IBPGR 1976; Ellis et al. 1985a, b; Hay and Whitehouse 2017). Seed dormancy is a difficult problem for genebanks that conserve a single crop and its wild relatives, but very much more so for genebanks that conserve diverse species across several plant families.
The remarkably brief estimate of seed storage longevity for Phleum pratense L. in the MTS (Fig. 4a, Table 2) is surprising. Canode (1972) reported that seed viability was not reduced in P. pratense after storage for 10 years at 5 °C and 40% relative humidity (about 7–8% moisture content, and so only slightly cooler but a little more moist than in the MTS). MacKay and Flood (1969) provided evidence of good survival of dried seeds of P. pratense stored in polythene bags in a basement in the UK (11 to 19 °C) over several years. In that study, samples at 7–9% moisture content showed little loss in viability over 78 months’ storage, whereas a sample at 12% moisture content showed reasonable survival for 66 months initially but viability then declined substantially during the subsequent 12 months. Interpolation from those results over the latter period suggests an estimate of around 1 year for σ at 12% moisture content with 11–19 °C. Moreover, seed storage conditions of 45 °C with 8% moisture content provided an estimate of σ of about 300 d for P. pratense (Ellis and Hong 2007b). This is quite similar to that of 281 d (Table 2) for the very much cooler and slightly drier storage regime of the MTS. Care is required to ensure that seed storage containers do provide a hermetic environment, because the effects of oxygen are particularly damaging to seed longevity at low moisture contents, including in P. pratense (Ellis and Hong 2007b). The analyses here were limited to only nine observations, whereas the research summarised above comprised considerably more observations. Given the contradiction between that literature, and see also Rincker (1981, 1983) and Rincker and Maguire (1979), and the results presented here (Fig. 4a; Table 2), we conclude that the latter are erroneous: the numbers of observations and seed lots analysed were limited, and many lots were not produced by ILRI but received as seeds without information on prior storage. We caution, therefore, that Table 2 may well overestimate loss in viability in P. pratense. This might have arisen from difficulties in assessing seed viability in germination tests, because its germination is particularly sensitive to the germination test media. P. pratense is often used in bioassays, because root and shoot growth is sensitive and easily damaged (e.g. Bari and Kato-Noguchi 2017). Similarly, seed germination in P. pratense is very sensitive to drought (e.g. Håkansson et al. 2013). These contrasting results illustrate the need for sufficient and accurate information on storage conditions, age of seeds, and sufficient numbers of germination test data points obtained from appropriate test environments when estimating genebank monitoring and regeneration intervals to avoid either loss of accessions from seed death during storage or unnecessary, high-risk, costly regeneration that risks the genetic integrity of accessions.
Extreme seed lots of Avena L., Sorghum, and Pennisetum also provided rapid loss in viability (Figs. 1, 2, 3). Two seed lots (out of 72) in Pennisetum showed loss in viability (Fig. 3) as rapid as P. pratense (Table 2), the four extreme seed lots in Sorghum were between P. pratense and Lolium L., whilst the extreme seed lot of Avena was similar to Digitaria Haller. We suggested previously that the minimum expectation for medium-term seed storage was > 2 years for a true loss in viability from 95 to 85% and noted that only 3 of the 19 genera of Fabaceae (where estimates could be provided) fell below this threshold in the MTS (Ellis et al. 2018). In Poaceae the equivalent comparison is only one (P. pratense) of 13 genera (Table 2). The extreme 2 seed lots of Pennisetum and the extreme four in Sorghum also fell below this threshold. Hence, fewer than 0.5% of the 4723 observations analysed provided results poorer than expected from medium-term storage.
The comparatively rapid loss in seed viability in certain grasses is recognised by ILRI. In addition to the long- and medium-term seed stores, a living collection (field genebank) of certain grasses (those known to have poor seed storage longevity) is also maintained. Fresh seeds are harvested regularly from this living collection for distribution and, if necessary, stored short-term in the MTS (and so monitored, albeit only until distributed).
Genebanks are responsible for the long-term conservation and also the distribution of germplasm (FAO 2014). The use of complimentary conservation strategies, as above, is a practical way to manage species with brief seed longevity. The storage of very short-lived seeds is neither practical nor economic for conservation, due to frequent monitoring and regeneration, in the context of the lifespan of perennial species. Frequent regeneration increases the risk of selection, genetic drift, and loss of genetic integrity and, hence, should be avoided (FAO 2014). Distribution of vegetative material has associated plant quarantine risks and requirements, and so seeds remain the preferred propagule for distribution. In such cases, maintaining plants in a secure field genebank is a good alternative for conservation when complimented by storage of minimum quantities of seeds to provide a readily-available supply for distribution and as backup to avoid loss of accessions in case of inclement weather, pests and diseases in the field. It is also of economic benefit in those cases where maintaining perennials in a field genebank costs less than regeneration, harvesting, cleaning, processing and entering seeds into storage on a frequent basis. Long-term seed storage remains the most cost-effective and secure method of germplasm conservation when possible. Future decisions on which grass accessions should be maintained in ILRI’s field genebank and which can safely be stored as seeds will be informed by the current results.
In order to distribute high-quality seed from genebanks, it was suggested originally that accessions be regenerated once seed lot viability had declined during storage to 85% (IBPGR 1976). Recognising that 85% may be difficult for all accessions entering a genebank, particularly with wild or forest tree species, this standard was later relaxed to 85% of the initial viability on entry into store (FAO/IPGRI 1994; FAO 2014). So, for example, if the initial viability of an accession were only 70% then the regeneration standard for that accession would be set at 60% in absolute terms.
Many of the initial tests on seed lots upon receipt by the MTS at ILRI provided very low values, often below 50% germination. This can be seen most easily where seed dormancy was initially high (Fig. 5), but also occurred in some seed lots with little or no dormancy. In Cenchrus L., for example, ability to germinate upon entry into the MTS varied between extremes of 1 and 99% amongst the 128 different seed lots. Hence, the relaxation of the regeneration standard to 85% of the value of viability on entry into a store was necessary, albeit necessitating very good seedbed environments in which to generate sufficient strong seedlings from low viability accessions (e.g. Khah et al. 1986; Ellis and Dolman 1988).
The consequences of the relaxation of the standard for genetic damage during genebank storage were not stated in the published standards (FAO/IPGRI 1994; FAO 2014). In cases where seed lot viability is low on entry into the genebank, it could be argued that considerable damage to genetic integrity has occurred already before receipt. If so, perhaps further deterioration in storage under good conditions might not damage genetic integrity that much more?
This is speculation because research on genetic integrity during seed storage has concentrated on high-quality seed lots. Nevertheless, the above reveals a gap in the tools available to genebank managers: conversion from estimated monitoring intervals of the fixed type shown in Table 2 where an 85% regeneration standard and an initial viability of 95% is assumed, towards more flexible advice covering different combinations of initial accession viability and regeneration standards. Table 3 provides an approach to support this task. The units therein are change in probit viability for various combinations of initial seed viability and regeneration standard. Multiplying these values by the estimate of σ in Table 2, for example, would provide the estimated period in years for a given reduction in viability. For example, if initial viability is 70% and the regeneration standard for that accession 60% (the example given above where the regeneration standard is 85% of the initial value); the change in probit viability is 0.27 (Table 3); if σ were, for example, 15.63 years (the value derived for Paspalum in the MTS, Table 2), then the estimated regeneration interval would be 4.22 years (= 0.27 × 15.63); and the monitoring interval 1.4 years (= 4.22/3). The results from long-term monitoring studies, such as reported here, provide important information for evidence-based management decisions for conserving forage germplasm.
References
Abdalla FH, Roberts EH (1968) Effects of temperature, moisture, and oxygen on the induction of chromosome damage in seeds of barley, broad bean and peas during storage. Ann Bot 32:119–136
Abdalla FH, Roberts EH (1969) The effects of temperature and moisture on the induction of genetic changes in seeds of barley, broad beans and peas during storage. Ann Bot 33:153–167
Aniszewski T, Haikonen J, Helwig B, Konert G, Oleksińska Z, Stenman A, Ylinampa T (2012) Vigor, vitality and seed dormancy of Avena sativa cultivars in a long-term experiment. J Appl Bot Food Qual 85:150–158
Bari IN, Kato-Noguchi H (2017) Phytotoxic effects of Cerbera manghas L. leaf extracts on seedling elongation of four monocot and four dicot test species. Acta Agrobot 70:1720
Canode CL (1972) Germination of grass seed as influenced by storage condition. Crop Sci 12:79–80
Ellis RH, Dolman GFS (1988) The germination and emergence of seeds of winter oilseed rape stored and sown in a mixture with pelleted methiocarb. Ann App Biol 112:555–561
Ellis RH, Hong TD (2007a) Quantitative response of the longevity of seed of twelve crops to temperature and moisture in hermetic storage. Seed Sci Technol 35:432–444
Ellis RH, Hong TD (2007b) Seed longevity – moisture content relationships in hermetic and open storage. Seed Sci Technol 35:423–431
Ellis RH, Roberts EH (1980) Improved equations for the prediction of seed longevity. Ann Bot 45:13–30
Ellis RH, Roberts EH (1981) The quantification of ageing and survival in orthodox seeds. Seed Sci Technol 9:373–409
Ellis RH, Osei-Bonsu K, Roberts EH (1982) The influence of genotype, temperature and moisture on seed longevity in chickpea, cowpea and soyabean. Ann Bot 50:69–82
Ellis RH, Hong TD, Roberts EH (1985a) Handbook of seed technology for genebanks. Volume I. Principles and methodology. International Board for Plant Genetic Resources, Maccarese
Ellis RH, Hong TD, Roberts EH (1985b) Handbook of seed technology for genebanks. Volume II. Compendium of specific germination information and test recommendations. International Board for Plant Genetic Resources, Maccarese
Ellis RH, Nasehzadeh M, Hanson J, Woldemariam Y (2018) Medium-term seed storage of 50 genera of forage legumes and evidence-based genebank monitoring intervals. Genet Resources Crop Evol 65:607–623
FAO (2014) Genebank standards for plant genetic resources for food and agriculture. Food and Agriculture Organization of the United Nations, Maccarese
FAO/IPGRI (1994) Genebank standards. Food and Agriculture Organization of the United Nations/International Plant Genetic Resources Institute, Rome
Håkansson I, ArvidssonJJ EtanaA, Rydborg T, Keller T (2013) Effects of seedbed properties on crop emergence. 6. Requirements of crops with small seeds. Acta Agric Scand B 63:554–563
Hay FR, Whitehouse KJ (2017) Rethinking the approach to viability monitoring in seed genebanks. Conserv Physiol 5(1):cox009. https://doi.org/10.1093/conphys/cox009
IBPGR (1976) Report of IBPGR working group on engineering, design and cost aspects of long-term seed storage facilities. International Board for Plant Genetic Resources, Maccarese
ISTA (2013) International rules for seed testing. International Seed Testing Association, Bern
Khah EM, Ellis RH, Roberts EH (1986) Effects of laboratory germination, soil temperature and moisture content on the emergence of spring wheat. J Agric Sci 107:431–438
MacKay DB, Flood RJ (1969) Investigations in crop seed longevity. III. The viability of grass and clover seed stored in permeable and impermeable containers. J Natn Inst Agric Bot 11:521–546
Nagel M, Vogel H, Landjeva S, Buck-Sorlin G, Lohwasser U, Scholz U, Börner A (2009) Seed conservation in ex situ genebanks—genetic studies on longevity in barley. Euphytica 170:5–14
Rincker CM (1981) Long-term subfreezing storage of forage crop seeds. Crop Sci 21:424–427
Rincker CM (1983) Germination of forage crop seeds after 20 years of subfreezing storage. Crop Sci 23:229–231
Rincker CM, Maguire JD (1979) Effect of seed storage on germination and forage production of seven grass cultivars. Crop Sci 19:857–860
Roos EE (1988) Genetic changes in a collection over time. HortSci 23:86–90
van Treuren R, Bas N, Kodde J, Groot SPC, Kik C (2018) Rapid loss of seed viability in ex situ conserved wheat and barley at 4°C as compared to −20°C storage. Conserv Physiol 6(1):coy033. https://doi.org/10.1093/conphys/coy033
Acknowledgements
We thank all the seed analysts at ILRI who carried out the germination tests which provided the data for our analyses, the German Bundesminister für Wirtschaftliche Zusammenarbeit (BMZ), the UK Department for International Development (DfID), the World Bank through their project on Upgrading the Genebanks of the Consultative Group on International Agricultural Research (CGIAR), the Global Crop Diversity Trust, and the CGIAR Research Program on Managing and Sustaining Crop Collections (Genebanks) for financial support.
Funding
In addition to the sources of funding listed, the authors are or were employees of the respective institutions listed here.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
No human participants or animals were involved in this research.
Informed consent
This manuscript is original and submitted with the consent of all authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ellis, R.H., Nasehzadeh, M., Hanson, J. et al. Medium-term seed storage of diverse genera of forage grasses, evidence-based genebank monitoring intervals, and regeneration standards. Genet Resour Crop Evol 66, 723–734 (2019). https://doi.org/10.1007/s10722-019-00748-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-019-00748-y