Abstract
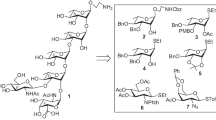
A concise synthetic strategy has been developed for the synthesis of the pentasaccharide repeating unit of the cell wall O-antigen of Escherichia coli O43 strain involving stereoselective β-D-mannosylation and α-L-fucosylation using corresponding trichloroacetimidate intermediates and perchloric acid supported over silica (HClO4-SiO2) as glycosylation promoter. The yield and stereoselectivity of the glycosylations were very good.





Similar content being viewed by others
References
Mokomane, M., Kasvosve, I., de Melo, E., Pernica, J.M., Goldfarb, D.M.: The global problem of childhood diarrhoeal diseases: emerging strategies in prevention and management. Ther. Adv. Infect, Dis. 5, 29–43 (2018)
Ashbolt, N.J.: Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology. 198, 229–238 (2004)
Dekker, J., Frank, K.: Salmonella, Shigella, and Yersinia. Clin. Lab. Med. 35, 225–246 (2015)
Crump, J.A., Sjölund-Karlsson, M., Gordon, M.A., Parry, C.M.: Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella Infections. Clin. Microbiol. Rev. 28, 901–937 (2015)
Morris Jr., J.G., Acheson, D.: Cholera and other types of vibriosis: a story of human pandemics and oysters on the half Shell. Clin. Infect. Dis. 37, 272–280 (2003)
Clements, A., Young, J.C., Constantinou, N., Frankel, G.: Infection strategies of enteric pathogenic Escherichia coli. Gut Microbes. 3, 71–87 (2012)
Acheson, D., Allos, B.M.: Campylobacter jejuni infections: update on emerging issues and trends. Clin. Infect. Dis. 32, 1201–1206 (2001)
Orskov, I., Orskov, F., Jann, B., Jann, K.: Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacterial Rev. 41, 667–710 (1977)
Kaper, J.B., Nataro, J.P., Mobley, H.L.T.: Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2, 123–140 (2004)
Johnson, J.R., Russo, T.A.: Extraintestinal pathogenic Escherichia coli : “the other bad E coli ”. J. Lab. Clin. Med. 139, 155–162 (2002)
Kelly, P.: Infectious diarrhoea. Medicine. 39, 201–206 (2011)
Stenutz, R., Weintraub, A., Widmalm, G.: The structures of Escherichia coli O-polysaccharide antigens. FEMS Microbiol. Rev. 30, 382–403 (2006)
Paton, J.C., Paton, A.W.: Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 11, 450–479 (1998)
Nataro, J.P., Kaper, J.B.: Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11, 142–201 (1998)
Perepelov, A.V., Guo, X., Filatov, A.V., Liu, B., Knirel, Y.A.: Structure and gene cluster of the O-antigen of Escherichia coli O43. Carbohydr. Res. 416, 32–36 (2015)
Roy, R.: New trends in carbohydrate based vaccines. Drug Discov. Today Technol. 1, 327–336 (2004)
Hölemann, A., Seeberger, P.H.: Carbohydrate diversity: synthesis of glycoconjugates and complex carbohydrates. Curr. Opin. Biotechnol. 15, 615–622 (2004)
Pozsgay, V.: Recent developments in synthetic oligosaccharide-based bacterial vaccines. Curr. Top. Med. Chem. 8, 126–140 (2008)
Morelli, L., Poletti, L., Lay, L.: Carbohydrates and immunology: synthetic oligosaccharide antigens for vaccine formulation. Eur. J. Org. Chem. 5723–5777 (2011)
Lee, C.-J., Lee, L.H., Frasch, C.E.: Protective immunity of pneumococcal glycoconjugates. Crit. Rev. Microbiol. 29, 333–349 (2003)
Cheesman, M.J., Ilanko, A., Blonk, B., Cock, I.E.: Developing new antimicrobial therapies: are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacogn. Rev. 11, 57–72 (2017)
He, H., Chen, D., Li, C., Zhao, J.-H., Qin, H.-B.: Synthesis of trisaccharide repeating unit of fucosylated chondroitin sulfate. Org. Biomol. Chem. 17, 2877–2882 (2019)
Susanto, W., Kong, K.-H., Hua, K.-F., Wu, S.-H., Lam, Y.: Synthesis of the trisaccharide repeating unit of capsular polysaccharide from Klebsiella pneumonia. Tetrahedron Lett. 60, 288–291 (2019)
Gucchait, Misra, A.K.: Influence of remote functional groups towards the formation of 1,2-cis glycosides: special emphasis on β-mannosylation. Org. Biomol. Chem. 17, 4605–4610 (2019)
Lönn, H.: Synthesis of a tri- and a heptasaccharide which contain α-L-fucopyranosyl groups and are part of the complex type of carbohydrate moiety of glycoproteins. Carbohydr. Res. 139, 105–113 (1985)
Schmidt, R.R., Zhu, X.: Glycosyl Trichloroacetimidates. In: Fraser-Reid, B.O., Tatsuta, K., Thiem, J. (eds.) Glycoscience. Springer, Berlin, Heidelberg (2008). https://doi.org/10.1007/978-3-540-30429-6_11
Chakraborti, A.K., Gulhane, R.: Perchloric acid adsorbed on silica gel as a new, highly efficient, and versatile catalyst for acetylation of phenols, thiols, alcohols, and amines. Chem. Commun. 2003, (1896-1897)
Mukhopadhyay, B., Maurer, S.V., Rudolph, N., van Well, R.M., Russell, D.A., Field, R.A.: From solution phase to “on-column” chemistry: trichloroacetimidate-based glycosylation promoted by perchloric acid-silica. J. Org. Chem. 70, 9059–9062 (2005)
Si, A., Misra, A.K.: Synthesis of a pentasaccharide repeating unit corresponding to the cell wall O-antigen of Escherichia coli O59 using iterative glycosylations in one pot. Tetrahedron. 72, 4435–4441 (2016)
Love, K.R., Andrade, R.B., Seeberger, P.H.: Linear synthesis of a protected H-type II pentasaccharide using glycosyl phosphate building blocks. J. Org. Chem. 66, 8165–8176 (2001)
DeNinno, M.P., Etienne, J.B., Duplantier, K.C.: A method for the selective reduction of carbohydrate 4,6-O-benzylidene acetals. Tetrahedron Lett. 36, 669–672 (1995)
Ishiwata, A., Munemura, Y., Ito, Y.: Synergistic solvent effect in 1,2-cis-glycoside formation. Tetrahedron. 64, 92–102 (2008)
Kanie, O., Ito, Y., Ogawa, T.: Orthogonal glycosylation strategy in oligosaccharide synthesis. J. Am. Chem. Soc. 116, 12073–12074 (1994)
Kasai, R., Okihara, M., Asakawa, J., Mizutani, K., Tanaka, O.: 13C NMR study of α- and β-anomeric pairs of D-mannopyranosides and L-rhamnopyranosides. Tetrahedron Lett. 35, 1427–1432 (1979)
Bock, K., Pedersen, C.: A study of 13CH coupling constants in hexopyranoses. J. Chem. Soc. Perkin Trans. 2, 293–297 (1974)
Wuts, P.G.M.: In: Crich, D. (ed.) Handbook of Reagents for Organic Synthesis: Reagents for Glycoside, Nucleotide, and Peptide Synthesis, pp. 425–428. Wiley, Chichester (2005)
Mukherjee, C., Misra, A.K.: Glycosylation and pyranose-furanose isomerization of carbohydrates using HClO4-SiO2: synthesis of oligosaccharides containing galactofuranose. Synthesis. 683–692 (2007)
Fügedi, P., Garegg, P.J.: A novel promoter for the efficient construction of 1,2-trans linkages in glycoside synthesis, using thioglycosides as glycosyl donors. Carbohydr. Res. 149, C9–C-12 (1986)
Veeneman, G.H., van Leeuwen, S.H., van Boom, J.H.: Iodonium ion promoted reactions at the anomeric centre. II An efficient thioglycoside mediated approach toward the formation of 1,2-trans linked glycosides and glycosidic esters. Tetrahedron Lett. 31, 1331–1334 (1990)
Lönn, H.: Glycosylation using a thioglycoside and methyl trifluoromethanesulfonate. A new and efficient method for cis and trans glycoside formation. J. Carbohydr. Chem. 6, 301–306 (1986)
Zheng, X., Xu, D., Edgar, K.J.: Cellulose levulinate: a protecting group for cellulose that can be selectively removed in the presence of other ester groups. Cellulose. 22, 301–311 (2015)
Bhattacharyya, S., Magnusson, B.G., Wellmar, U., Nilsson, U.J.: The p-methoxybenzyl ether as an in situ-removable carbohydrate-protecting group: a simple one-pot synthesis of the globotetraose tetrasaccharide. J. Chem. Soc. Perkin Trans. 1, 886–890 (2001)
Shangguan, N., Katukojvala, S., Greenberg, R., Williams, L.J.: The reaction of thio acids with azides: a new mechanism and new synthetic applications. J. Am. Chem. Soc. 125, 7754–7755 (2003)
Pearlman, W.M.: Noble metal hydroxides on carbon nonpyrophoric dry catalysts. Tetrahedron Lett. 8, 1663–1664 (1967)
Acknowledgements
P. S. thanks CSIR, New Delhi for providing Senior Research Fellowship. The work is supported by SERB, India (Project No. CRG/2019/000352 dated 23.01.2020) (AKM) and Bose Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shit, P., Misra, A.K. Straightforward synthesis of the pentasaccharide repeating unit of the cell wall O-antigen of Escherichia coli O43 strain. Glycoconj J 37, 647–656 (2020). https://doi.org/10.1007/s10719-020-09933-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-020-09933-z