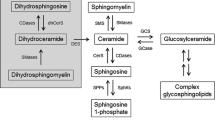
Abstract
Sphingolipid metabolism is an intricate network of several interdependent and co-regulated pathways. In addition to the mainstream biosynthetic and catabolic pathways, several processes, even if less important in contributing to the final tissue sphingolipid composition from the quantitative point of view, might become relevant when sphingolipid metabolism is for any reason dysregulated and concur to the onset of neuronal pathologies. The main subcellular sites involved in the mainstream metabolic pathway are represented by the Golgi apparatus (for the biosynthesis) and by the lysosomes (for catabolism). On the other hand, the minor collateral pathways are associated with the plasma membrane and membranes of other organelles, and likely play important roles in the local regulation of membrane dynamics and contribute to maintain a perfect membrane organization functional to the physiology of the cell. In this review, we will consider few aspects of the sphingolipid metabolic pathway depending by the dynamic of the membranes that seems to become relevant in neurodegenerative diseases.
Similar content being viewed by others
References
Ikeda, M., Kihara, A., Igarashi, Y.: Lipid asymmetry of the eukaryotic plasma membrane: functions and related enzymes. Biol. Pharm. Bull. 29(8), 1542–1546 (2006)
van Meer, G., Voelker, D.R., Feigenson, G.W.: Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9(2), 112–124 (2008)
Merrill Jr., A.H.: Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev. 111(10), 6387–6422 (2011). https://doi.org/10.1021/cr2002917
Simons, K., Ikonen, E.: Functional rafts in cell membranes. Nature. 387(6633), 569–572 (1997). https://doi.org/10.1038/42408
Sonnino, S., Prinetti, A., Mauri, L., Chigorno, V., Tettamanti, G.: Dynamic and structural properties of sphingolipids as driving forces for the formation of membrane domains. Chem. Rev. 106(6), 2111–2125 (2006)
Sonnino, S., Prinetti, A.: Membrane domains and the "lipid raft" concept. Curr. Med. Chem. 20(1), 4–21 (2013) CMC-EPUB-20121108-2 [pii]
Simons, K., Toomre, D.: Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1(1), 31–39 (2000). https://doi.org/10.1038/35036052
Prinetti, A., Loberto, N., Chigorno, V., Sonnino, S.: Glycosphingolipid behaviour in complex membranes. Biochim. Biophys. Acta. 1788(1), 184–193 (2009). https://doi.org/10.1016/j.bbamem.2008.09.001
Kitatani, K., Idkowiak-Baldys, J., Hannun, Y.A.: The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell. Signal. 20(6), 1010–1018 (2008)
Blott, E.J., Griffiths, G.M.: Secretory lysosomes. Nat. Rev. Mol. Cell Biol. 3(2), 122–131 (2002). https://doi.org/10.1038/nrm732
Baron, R., Neff, L., Louvard, D., Courtoy, P.J.: Cell-mediated extracellular acidification and bone resorption: evidence for a low pH in resorbing lacunae and localization of a 100-kD lysosomal membrane protein at the osteoclast ruffled border. J. Cell Biol. 101(6), 2210–2222 (1985)
Draeger, A., Schoenauer, R., Atanassoff, A.P., Wolfmeier, H., Babiychuk, E.B.: Dealing with damage: plasma membrane repair mechanisms. Biochimie. 107(Pt A), 66–72 (2014). https://doi.org/10.1016/j.biochi.2014.08.008
Reddy, A., Caler, E.V., Andrews, N.W.: Plasma membrane repair is mediated by ca(2+)-regulated exocytosis of lysosomes. Cell. 106(2), 157–169 (2001)
Rothman, J.E.: Mechanisms of intracellular protein transport. Nature. 372(6501), 55–63 (1994). https://doi.org/10.1038/372055a0
Rao, S.K., Huynh, C., Proux-Gillardeaux, V., Galli, T., Andrews, N.W.: Identification of SNAREs involved in synaptotagmin VII-regulated lysosomal exocytosis. J. Biol. Chem. 279(19), 20471–20479 (2004). https://doi.org/10.1074/jbc.M400798200
Andrews, N.W., Chakrabarti, S.: There's more to life than neurotransmission: the regulation of exocytosis by synaptotagmin VII. Trends Cell Biol. 15(11), 626–631 (2005). https://doi.org/10.1016/j.tcb.2005.09.001
Tucker, W.C., Chapman, E.R.: Role of synaptotagmin in Ca2+−triggered exocytosis. The Biochemical journal. 366(Pt 1), 1–13 (2002). https://doi.org/10.1042/BJ20020776
Arantes, R.M., Andrews, N.W.: A role for synaptotagmin VII-regulated exocytosis of lysosomes in neurite outgrowth from primary sympathetic neurons. J. Neurosci. Off. J. Soc. Neurosci. 26(17), 4630–4637 (2006). https://doi.org/10.1523/JNEUROSCI.0009-06.2006
Martinez-Arca, S., Alberts, P., Zahraoui, A., Louvard, D., Galli, T.: Role of tetanus neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP) in vesicular transport mediating neurite outgrowth. J. Cell Biol. 149(4), 889–900 (2000)
Martinez-Arca, S., Coco, S., Mainguy, G., Schenk, U., Alberts, P., Bouille, P., Mezzina, M., Prochiantz, A., Matteoli, M., Louvard, D., Galli, T.: A common exocytotic mechanism mediates axonal and dendritic outgrowth. J. Neurosci. Off. J. Soc. Neurosci. 21(11), 3830–3838 (2001)
Alberts, P., Rudge, R., Hinners, I., Muzerelle, A., Martinez-Arca, S., Irinopoulou, T., Marthiens, V., Tooze, S., Rathjen, F., Gaspar, P., Galli, T.: Cross talk between tetanus neurotoxin-insensitive vesicle-associated membrane protein-mediated transport and L1-mediated adhesion. Mol. Biol. Cell. 14(10), 4207–4220 (2003). https://doi.org/10.1091/mbc.e03-03-0147
Raiborg, C., Wenzel, E.M., Pedersen, N.M., Olsvik, H., Schink, K.O., Schultz, S.W., Vietri, M., Nisi, V., Bucci, C., Brech, A., Johansen, T., Stenmark, H.: Repeated ER-endosome contacts promote endosome translocation and neurite outgrowth. Nature. 520(7546), 234–238 (2015). https://doi.org/10.1038/nature14359
Aureli, M., Loberto, N., Bassi, R., Ferraretto, A., Perego, S., Lanteri, P., Chigorno, V., Sonnino, S., Prinetti, A.: Plasma membrane-associated glycohydrolases activation by extracellular acidification due to proton exchangers. Neurochem. Res. 37(6), 1296–1307 (2012). https://doi.org/10.1007/s11064-012-0725-1
Samarani, M., Loberto, N., Soldà, G., Straniero, L., Asselta, R., Duga, S., Lunghi, G., Zucca, F.A., Mauri, L., Ciampa, M.G., Schiumarini, D., Bassi, R., Giussani, P., Chiricozzi, E., Prinetti, A., Aureli, M., Sonnino, S.: A lysosome-plasma membrane-sphingolipid axis linking lysosomal storage to cell growth arrest. FASEB J. fj.201701512RR (2018). https://doi.org/10.1096/fj.201701512RR
Gabande-Rodriguez, E., Boya, P., Labrador, V., Dotti, C.G., Ledesma, M.D.: High sphingomyelin levels induce lysosomal damage and autophagy dysfunction in Niemann pick disease type a. Cell Death Differ. 21(6), 864–875 (2014). https://doi.org/10.1038/cdd.2014.4
Riboni, L., Viani, P., Bassi, R., Prinetti, A., Tettamanti, G.: The role of sphingolipids in the process of signal transduction. Prog. Lipid Res. 36(2–3), 153–195 (1997)
Sonnino, S., Cantu, L., Corti, M., Acquotti, D., Venerando, B.: Aggregative properties of gangliosides in solution. Chem. Phys. Lipids. 71(1), 21–45 (1994)
Koynova, R., Caffrey, M.: Phases and phase transitions of the sphingolipids. Biochim. Biophys. Acta. 1255(3), 213–236 (1995)
Rueda, R., Tabsh, K., Ladisch, S.: Detection of complex gangliosides in human amniotic fluid. FEBS Lett. 328(1–2), 13–16 (1993)
Cotterchio, M., Seyfried, T.N.: Serum gangliosides in mice with metastatic and non-metastatic brain tumors. J. Lipid Res. 35(1), 10–14 (1994)
Valentino, L.A., Ladisch, S.: Circulating tumor gangliosides enhance platelet activation. Blood. 83(10), 2872–2877 (1994)
Kloppel, T.M., Keenan, T.W., Freeman, M.J., Morre, D.J.: Glycolipid-bound sialic acid in serum: increased levels in mice and humans bearing mammary carcinomas. Proc. Natl. Acad. Sci. U. S. A. 74(7), 3011–3013 (1977)
Young Jr., W.W., Borgman, C.A., Wolock, D.M.: Modes of shedding of glycosphingolipids from mouse lymphoma cells. J. Biol. Chem. 261(5), 2279–2283 (1986)
Chigorno, V., Sciannamblo, M., Mikulak, J., Prinetti, A., Sonnino, S.: Efflux of sphingolipids metabolically labeled with [1-3H] sphingosine, L-[3-3H] serine and [9,10-3H] palmitic acid from normal cells in culture. Glycoconj. J. 23(3–4), 159–165 (2006). https://doi.org/10.1007/s10719-006-7921-7
Portoukalian, J., Zwingelstein, G., Abdul-Malak, N., Dore, J.F.: Alteration of gangliosides in plasma and red cells of humans bearing melanoma tumors. Biochem. Biophys. Res. Commun. 85(3), 916–920 (1978)
Olshefski, R., Ladisch, S.: Intercellular transfer of shed tumor cell gangliosides. FEBS Lett. 386(1), 11–14 (1996)
Li, R.X., Ladisch, S.: Shedding of human neuroblastoma gangliosides. Biochim. Biophys. Acta. 1083(1), 57–64 (1991)
Stephenson, J., Nutma, E., van der Valk, P., Amor, S.: Inflammation in CNS neurodegenerative diseases. Immunology 154(2), 204–219 (2018). https://doi.org/10.1111/imm.12922
Ladisch, S., Li, R., Olson, E.: Ceramide structure predicts tumor ganglioside immunosuppressive activity. Proc. Natl. Acad. Sci. U. S. A. 91(5), 1974–1978 (1994)
Grassi, S., Chiricozzi, E., Mauri, L., Sonnino, S., Prinetti, A.: Sphingolipids and neuronal degeneration in lysosomal storage disorders. J. Neurochem. (2018). https://doi.org/10.1111/jnc.14540
Vitner, E.B., Platt, F.M., Futerman, A.H.: Common and uncommon pathogenic cascades in lysosomal storage diseases. J. Biol. Chem. 285(27), 20423–20427 (2010). R110.134452 [pii]). https://doi.org/10.1074/jbc.R110.134452
Levine, T.P., Patel, S.: Signalling at membrane contact sites: two membranes come together to handle second messengers. Curr. Opin. Cell Biol. 39, 77–83 (2016). https://doi.org/10.1016/j.ceb.2016.02.011
Prinz, W.A.: Bridging the gap: membrane contact sites in signaling, metabolism, and organelle dynamics. J. Cell Biol. 205(6), 759–769 (2014). https://doi.org/10.1083/jcb.201401126
Levine, T., Loewen, C.: Inter-organelle membrane contact sites: through a glass, darkly. Curr. Opin. Cell Biol. 18(4), 371–378 (2006). https://doi.org/10.1016/j.ceb.2006.06.011
Gatta, A.T., Levine, T.P.: Piecing together the patchwork of contact sites. Trends Cell Biol. 27(3), 214–229 (2017). https://doi.org/10.1016/j.tcb.2016.08.010
Jain, A., Holthuis, J.C.M.: Membrane contact sites, ancient and central hubs of cellular lipid logistics. Biochim. Biophys. Acta. 1864(9), 1450–1458 (2017). https://doi.org/10.1016/j.bbamcr.2017.05.017
Matarrese, P., Garofalo, T., Manganelli, V., Gambardella, L., Marconi, M., Grasso, M., Tinari, A., Misasi, R., Malorni, W., Sorice, M.: Evidence for the involvement of GD3 ganglioside in autophagosome formation and maturation. Autophagy. 10(5), 750–765 (2014). https://doi.org/10.4161/auto.27959
Sano, R., Annunziata, I., Patterson, A., Moshiach, S., Gomero, E., Opferman, J., Forte, M., d'Azzo, A.: GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to ca(2+)-dependent mitochondrial apoptosis. Mol. Cell. 36(3), 500–511 (2009). https://doi.org/10.1016/j.molcel.2009.10.021
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Aureli, M., Samarani, M., Loberto, N. et al. Neuronal membrane dynamics as fine regulator of sphingolipid composition. Glycoconj J 35, 397–402 (2018). https://doi.org/10.1007/s10719-018-9841-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-018-9841-8