Abstract
Cancer is a major cause of death in both developing and developed countries. Early detection and efficient therapy can greatly enhance survival. Aberrant glycosylation has been recognized to be one of the hallmarks of cancer as glycans participate in many cancer-associated events. Cancer-associated glycosylation changes often involve sialic acids which play important roles in cell-cell interaction, recognition and immunological response. This review aims at giving a comprehensive overview of the literature on changes of sialylation in serum of cancer patients. Furthermore, the methods available to measure serum and plasma sialic acids as well as possible underlying biochemical mechanisms involved in the serum sialylation changes are surveyed. In general, total serum sialylation levels appear to be increased with various malignancies and show a potential for clinical applications, especially for disease monitoring and prognosis. In addition to overall sialic acid levels and the amount of sialic acid per total protein, glycoprofiling of specific cancer-associated glycoproteins, acute phase proteins and immunoglobulins in serum as well as the measurements of sialylation-related enzymes such as sialidases and sialyltransferases have been reported for early detection of cancer, assessing cancer progression and improving prognosis of cancer patients. Moreover, sialic-acid containing glycan antigens such as CA19–9, sialyl Lewis X and sialyl Tn on serum proteins have also displayed their value in cancer diagnosis and management whereby increased levels of these factors positively correlated with metastasis or poor prognosis.
Similar content being viewed by others
Introduction
Cancer, an increasing burden worldwide, is a major cause of death in both developing and developed countries. In 2012, about 14.1 million new cancer cases and 8.2 million deaths from cancer are estimated to have occurred worldwide [1, 2]. Understanding the complex cancer biology and employing reliable biomarkers for detecting and staging malignant diseases and for evaluating various therapeutic approaches can facilitate early detection, efficient therapy and prognosis of cancer and may thereby greatly enhance survival [3,4,5]. Glycosylation is an important and prevalent modification of proteins and lipids, and is involved in numerous key physiological and pathological processes including malignant transformation, cancer progression and metastasis [6,7,8,9]. Aberrant glycosylation has been recognized to be one of the hallmarks of cancer as glycans participate in many cancer-associated events such as cell differentiation, migration, adhesion, invasion, metastasis, cell signaling and trafficking [5, 9,10,11,12,13]. Exploiting differences in glycosylation between malignant and healthy individuals therefore offers opportunities to reveal aspects of the complex cancer biology and to identify more sensitive and specific cancer biomarkers.
The two main types of glycans are N-linked and O-linked glycans (Fig. 1), which in mammals are composed of the building blocks N-acetylglucosamine, galactose, N-acetylgalactosamine, fucose, mannose, and sialic acid, and are present on most proteins in human cells and blood circulation [14,15,16]. Numerous studies have shown that changes in serum/plasma glycan structures occur during cancer initiation, progression, and treatment. This makes glycan markers from serum/plasma a promising, non-invasive group of novel biomarkers for diagnosis, prognosis, and treatment monitoring [10, 17, 18]. Changes in serum N- and O-linked glycan structures occur not only on cancer-derived cells and proteins, but also on B lymphocyte-derived immunoglobulins and liver-synthesized acute phase proteins such as haptoglobin, α-1-antitrypsin and α-1-acid glycoprotein. This suggests that altered glycosylation may be the result of a systemic tumor response. Therefore, glycans are potentially suitable biomarkers associated with system malfunction in the blood circulation of cancer patients [19,20,21,22,23].
Sialic acids are an important group of monosaccharides with regard to cancer-associated glycan changes. The most abundant forms of this sugar are derivatives of the neuraminic acid, consisting of a nine-carbon backbone, a carboxyl group, and an amino group that is substituted by either an acetyl or glycolyl group (Fig. 2). The most common sialic acid derivative in mammals is N-acetylneuraminic acid (Neu5Ac). Another derivative is N-glycolylneuraminic acid (Neu5Gc) [24, 25]. However, humans are unable to synthesize Neu5Gc as a consequence of genomic mutations. The biosynthesis of Neu5Gc arises from the action of a hydroxylase that converts the nucleotide donor cytidine monophosphate N-acetylneuraminic acid (CMP-Neu5Ac) to cytidine monophosphate N-glycolylneuraminic acid (CMP-Neu5Gc). This enzymatic activity is present in animal cells, but not in human cells due to a partial deletion in the gene that encodes CMP-Neu5Ac hydroxylase [26, 27]. For simplicity, the generic term ‘sialic acid’ is used throughout this review when referring to N-acetylneuraminic acid unless stated otherwise.
Sialic acids are abundant on various glycoproteins and glycolipids (gangliosides) and are usually terminally attached to the end of the glycan. This forms an outer layer on the cell membrane and glycoconjugates. Sialic acids are linked to other sugars such as galactose or N-acetylhexosamine via α2–3- or α2–6-glycosidic bonds or through poly-sialic acid repeats in α2–8-linkage, all catalyzed by specific enzymes. Furthermore, sialic acids exist with different modifications in which the hydroxyl groups may either be methylated or esterified with acetyl, lactyl, phosphate, or sulfate groups (Fig. 2) [28].
As a result of their location, ubiquitous distribution and unique structural features, sialic acids can mediate a wide variety of physiological and pathological processes. They play a role in cellular functions such as transport of positively charged compounds, cellular interaction, conformational changes of glycoproteins on cell membranes, and even masking cell surface antigens [25]. Aberrant sialylation has been implicated in the disturbance of cell-cell recognition, cell adhesion, antigenicity, protein targeting and invasion [29,30,31,32,33,34,35]. Studies of malignant cells have revealed alterations in cell surfaces and membranes in terms of the sialic acid content of glycoproteins and glycolipids; accordingly, increased sialylation is one of the main characteristics of malignant transformation [36,37,38,39,40,41,42]. Notably, the glycoproteins and glycolipids expressed by tumors can be released into the serum through increased turnover, secretion, and/or shedding [43, 44].
The documentation of alterations in the turnover and release of tumor cell surface glycoconjugates have stimulated investigators’ interest in the measurement and evaluation of serum/plasma sialoglycoproteins and sialoglycolipids. Several investigators have studied their levels including different forms of sialic acids in the serum or plasma of patients with malignant disease. Unlike tumor antigens, which are associated with a limited spectrum of tumors, increased sialic acid levels appear to be a common phenomenon of a variety of neoplastic cells and have been traced down to enhanced sialyltransferase activity, reduced sialidase activity and/or increased sialylglycoprotein production [25, 45]. As a result, monitoring serum/plasma factors such as overall sialic acid content, sialidase activity and sialyltransferase expression, as well as sialylation changes on specific serum glycoproteins may have useful clinical applications for the detection, staging and prognosis of different diseases and cancers as reviewed in the following. In addition, the methods available to measure serum/plasma sialic acid as well as possible underlying biochemical mechanisms involved in the serum/plasma sialylation changes are discussed.
Overall serum sialic acid levels as markers for malignancy
The first sialic acid measurements were made by Winzler in 1958, MacBeth and Bekesi in 1962 and continued by Brozmanova 10 years later [46,47,48]. These studies primarily focused on the overall sialic acid levels as total sialic acid (TSA) content which included glycoprotein- and glycolipid-bound sialic acids, and small amounts of free sialic acid, as well as glycolipid-bound sialic acids (LSA) only. Between 98% and 99.5% of TSA found in serum and plasma is bound to glycoproteins and only a small fraction of the sialic acids is bound to lipids, mostly in the form of gangliosides [31]. Normal serum TSA levels of a healthy individual are in the range of 51 to 84 mg/dl, while the lipid fraction only accounts for 0.4 to 0.9 mg/dl of sialic acids [49]. Increased serum TSA, LSA or normalized sialic acid levels such as TSA/total protein (TP) and bound sialic acids/TP were discovered in different types of cancer and have repeatedly been reported to have potential for cancer diagnosis, staging and prognosis.
Shah et al., for example, evaluated serum TSA by a spectrophotometric method as well as linkage-specific sialylation via lectins in the serum of oral cancer patients and controls. Cancer patients were followed up after initiation of anticancer treatment and the patients’ response to the anticancer treatment was assessed. They found significantly higher serum levels of TSA and TSA/TP in oral pre-cancerous conditions as compared to healthy controls. In addition to higher serum levels of TSA and TSA/TP, specifically α2–6-sialylation was found to be increased in untreated oral cancer patients as compared to healthy controls, oral pre-cancerous conditions and responders, while levels of these markers in non-responders were comparable to that of untreated patients. Furthermore, serum α2–3-sialylation levels of non-responders were higher than those of responders. Overall, these sialylation changes in serum correlated to neoplastic transformation and disease progression [50]. In line with this, Sawhney et al. evaluated the usefulness of serum TSA and serum LSA as markers for the early detection and staging of oral cancer by spectrophotometric method. This study confirmed that serum TSA and LSA levels were significantly elevated in oral pre-cancer and cancer patients when compared to healthy controls, and progressively increased with grades of dysplasia in precancerous groups and with the extent of malignant disease (TNM Clinical staging) as well as histopathological grades in the cancer group. Serum LSA, in particular, appeared to show potential clinical utility in predicting premalignant changes [51]. Accordingly, the diagnostic potential of overall serum sialic acids as markers in oral cancer has been reported in several other studies [52,53,54,55,56,57]. Furthermore, total serum sialic acid levels have been shown to alter in ovarian cancer [58], cholangiocarcinoma [59], cervical cancer [60], leukemia [61], colorectal cancer [62, 63], breast cancer [64] and lung cancer [65] which has pointed towards their clinical usefulness as a potential diagnostic and/or prognostic tumor marker. Similarly, Dwivedi et al. showed that plasma LSA could be useful as a prognostic determinant in a variety of neoplastic conditions (breast cancer, lung cancer, colon cancer, ovarian cancer, prostate cancer, leukemia, gastrointestinal, thyroid cancer, pancreas cancer and adrenal cancer patients) with high sensitivity [66]. Another study by Tewarson et al. determined the serum sialylation in cancer patients of stomach, breast, colorectal region and gall bladder with varying degrees of metastasis before and after treatment as well as in healthy controls. Results showed that serum TSA and TSA/TP levels were significantly elevated in all cases of cancer which associated with the degree of metastasis. The disease-associated elevation of TSA/TP reversed to a certain extent after effective therapy [67]. The prognostic value of serum TSA or LSA in stomach cancer [68], thyroid cancer [69], colorectal cancer [70, 71] breast cancer [72] and malignant melanoma [73,74,75] was also confirmed by other groups.
Importantly, as the elevation of serum/plasma TSA and TSA/TP was observed for several cancers, it seems more promising as a prognostic and therapy efficiency marker where the requirement for cancer specificity is less than for diagnosis. Nevertheless, determining the sialic acid content in addition to more cancer-specific markers may enhance the performance of current markers. With regard to cancer specificity, Plucinsky et al. investigated serum TSA in cancer patients with various primary sites (rectal, melanomas, breast, gastrointestinal and pancreatic), nonmalignant diseases (villous adenomas, ulcerative colitis, hernias, endocrine disease, intestinal disease, liver disease, breast disease and regional enteritis) and healthy controls. Data analysis indicated significant increases in the average serum TSA levels in cancer and benign diseases in comparison with healthy controls. In the groups of cancer, rectal cancer showed the lowest and pancreatic showed the highest average levels of serum TSA. In the group of the benign diseases, villous adenoma patients displayed the lowest and regional enteritis recorded the highest mean value of serum TSA [76].
With regard to cancer marker potential, serum free sialic acid (FSA) as well as tissue TSA did not appear to be as conclusive as serum bound sialic acids/TSA in several types of cancer such as laryngeal cancer [30], endometrial cancer [77] and colorectal cancer [63]. In addition, serum bound sialic acid contents showed no correlation to tissue sialic acid levels in colon cancer [63]. Specifically, studies of Kim and coworkers as well as Dall’Olio et al. reported decreased TSA/TP content in human colonic tumors compared to the level in normal tissue from the same patients [78,79,80].
In contrast to this, some studies reported conflicting results. Romppanen et al. revealed that the elevation of serum TSA, TSA/TP and LSA concentrations in breast cancer had low sensitivities and low accuracy in differentiating between breast cancer and benign breast disease since both pathologies caused an increase of serum TSA, TSA/TP and LSA [81]. Another study by Vivas et al. also indicated that serum TSA or LSA seem to have little value for the early detection of cervical cancer or clinical staging (sensitivity for stage IB 0% for TSA, 27% for LSA) as serum TSA and LSA concentrations in patients with cervical cancer were only found to correlate with advanced-stage disease [82]. In addition, serum LSA/TSA measurement was found not to be useful for detecting early-stage colorectal cancer (Dukes A and B) [83], while TSA normalized to TP (TSA/TP) showed potential in early detection of colorectal cancer and follow-up of patients during treatment [71].
One may conclude from the aforementioned studies that overall sialic acid levels in serum or plasma as markers appear to show good sensitivity for various types of cancer. However, overall serum sialic acid levels are also elevated in some benign and inflammatory conditions which illustrates some lack of cancer-specificity, thereby limiting their use for early detection and cancer screening. Furthermore, there is an insufficient sample size (size range: 2–1280, median: 121, interquartile range: 73–289) of some studies and only few validation studies have been performed in an independent validation cohort from the studies mentioned above (Table 1). These may be some of the reasons why the interest in exploring overall serum sialic acid levels as a cancer marker has failed to develop further. Overall serum sialic acid measurements might, however, warrant further evaluation in combination with the measurement of existing markers for improved performance in cancer diagnosis, cancer staging, and monitoring of therapeutic response in several types of cancer.
Protein-specific sialylation changes as serum markers in specific cancer
Studies on the utility of overall serum sialic acid levels as a cancer marker have indicated the importance of identifying cancer-specific markers, especially with regard to diagnosis. The concern over disease-specificity may be addressed through the analysis of individual sialylated glycoproteins, as reviewed in the following.
It is well known that the oncogenic process results in significant alterations of the cellular glycosylation pattern. Glycoproteins secreted by tumors may reflect the altered glycosylation machinery of cancer cells and can be detected in physiological fluids [84]. Potential tumor biomarkers may be identified based on both changes of the protein glycosylation and protein concentrations [85, 86]. In prostate cancer, determining the concentration of prostate specific antigen (PSA) alone has displayed limitations in early detection [87].PSA-specific glycosylation changes in serum from prostate cancer patients compared with controls have been characterized by employing matrix-assisted laser desorption/ionization-time-of-flight-mass spectrometry (MALDI-TOF-MS); the levels of α2–3-linked sialic acids on PSA illustrated great potential in discriminating malignant from benign conditions, thereby improving prostate cancer diagnosis [88]. Recently, Pihikova et al. analyzed prostate cancer serum samples applying a new electrochemical label-free method [89]. Maackia amurensis agglutinin (MAA, a lectin recognizing α2–3-terminal sialic acids) binding to serum PSA was significantly higher (5.3-fold) for prostate cancer samples than for healthy controls, suggesting that a combined analysis of serum PSA levels and glycoprofiling of PSA has a potential for improved detection of prostate cancer [89]. Accordingly, Llop et al. also evaluated the portion of α2–3-sialylated PSA in serum with a lectin immunoaffinity column and revealed that α2–3-sialic acid on PSA exhibited high performance in discriminating between high-risk prostate cancer patients and the benign prostate hyperplasia individuals [90]. In addition, the differentiation between aggressive and non-aggressive prostate cancer based on PSA α2–3-sialylation showed high sensitivity, specificity and accuracy, which indicated that α2–3-sialic acid content on PSA can improve prostate cancer diagnosis and clinical decision making [90]. Similarly, Yoneyama et al. measured serum α2–3-sialylated PSA using a magnetic microbead-based immunoassay in a training (n = 100) and a validation set (n = 314) of prostate cancer samples and suggested that α2–3-sialylated PSA may improve the accuracy of prostate cancer early detection [91]. With regard to other cancer types, altered glycosylation of serum MUC1, a highly sialylated glycoprotein, exhibited potential for the early diagnosis of breast cancer [92] and sialylation of serum MUC1 was also enhanced in colorectal cancer patients [93].
Another change in the protein metabolism of cancer cells is the elevated hepatic production of acute phase proteins which may play an important role in cancer pathologies. Wu et al. identified and validated differentially expressed sialoglycoproteins in the serum of ovarian cancer patients using a lectin-based ELISA assay and quantitative glycoproteomics analysis in three serum sample sets including one discovery set (n = 34) and two validation sets (validation 1: n = 83; validation 2: n = 88) and found that the sialoglycoproteins clusterin (CLUS), leucine-rich alpha-2-glycoprotein (LRG1), hemopexin (HEMO), vitamin D-binding protein (VDB), and complement factor H (CFH) were differentially expressed in the serum of ovarian cancer patients compared to benign diseases. Moreover, the decreased sialylation levels of CLUS, CFH, and HEMO in serum of ovarian cancer patients were validated which showed that these biomarkers have potential utility for diagnosis of ovarian cancer with high accuracy [94]. Kontro et al. found that pancreatic cancer and acute pancreatitis were related to changes of serum concentrations of sialylated glycoproteins derived from acute phase proteins and immunoglobulins by ultra-high-performance liquid chromatography (UPLC)-mass spectrometry. Pancreatitis patients showed 38 changes of site-specific glycoforms of sialylated serum glycoproteins as compared to healthy controls, whilst in pancreatic cancer patients 13 such changes as compared to healthy controls were observed, showing the potential of these glycoform changes for pancreatic cancer detection [95]. Zhao et al. developed a strategy for evaluating sialylated glycoprotein markers in human cancer by combining lectin assays with mass spectrometric analysis. Employing this method, approximately 130 sialylated glycoproteins were identified and sialylated plasma protease C1 inhibitor was found to be down-regulated in pancreatic cancer serum, which may serve as a marker for cancer [96]. Another study in small cell and non-small cell lung cancer patients showed different glycosylation profiles including α2–3-sialylation and expression of sialyl Lewis X (SLX) epitopes on acute phase proteins of α-1-acid glycoprotein and haptoglobin [97].
Recently, emerging evidence indicates that altered immunoglobulin G (IgG) glycosylation is associated with various diseases including cancer [98, 99]. There has been an increasing interest in the analysis serum IgG glycoforms [100]. Decreased IgG sialylation levels, which associated with cancer pathogenesis or poorer prognosis, were observed in different types of cancer such as colorectal cancer [101, 102], gastric cancer [99, 103] and ovarian cancer [104]. In contrast, increased IgG sialylation was found to be linked to higher risk of multiple myeloma [105], which indicated different mechanisms involved in tumor pathologies leading to cancer-type-specific IgG sialylation changes. Decreased IgG sialylation was also described as a pro-inflammatory signal [106, 107]. The addition of sialic acid to IgG glycans has been shown to convert IgG from a pro-inflammatory to an anti-inflammatory status and, consequently, sialic acids on IgG are believed to be essential for the anti-inflammatory effect in intravenous immunoglobulin therapy [108].
Evaluating glycosylation changes on specific glycoproteins seems to be one of the most promising approaches to identify cancer-specific markers. Nevertheless, though the aforementioned results showed great potential for sialylation changes on specific glycoproteins to serve as novel or improved markers, only a few studies mentioned above were validated. Larger studies are still required for the translation of these markers from the lab to the clinics.
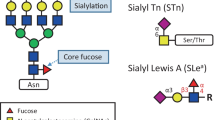
Utility of serum/plasma sialyl Lewis A (CA19–9), sialyl Lewis X (SLX) and sialyl Tn (STN) antigens as markers in cancer
Many tumor associated antigens which can be detected by available monoclonal antibodies are glycoconjugates on the cell surface [109]. Of the most common tumor-associated carbohydrate structures, sialyl Lewis A (CA19–9) and sialyl Lewis X (SLX) antigens are examples of type 1 and type 2 terminal carbohydrate structures, respectively, and sialyl Tn antigen (STN) is an example of an O-glycan core structure (Fig. 3). While CA19–9 and SLX both play an important role in cancer metastasis as ligands for endothelial cell E-selectin responsible for cell adhesion [110,111,112,113,114], they are also found on proteins in serum of cancer patients. Serum CA19–9, for example, showed great value as marker in the management of pancreatic cancer and is currently used in the clinic [115]. Furthermore, it displayed great potential as a diagnostic marker and predictor for metastasis in colorectal cancer [116]. High preoperative serum levels of CA19–9 and SLX have been shown to be predictive for poor prognosis of colorectal cancer after surgery [117,118,119,120,121,122,123]. Moreover, high preoperative serum levels of CA19–9, SLX and STN were found to be associated with liver metastasis in gastric cancer [124]. Besides this, the determination of preoperative serum STN level may also be a useful tool as a predictor of distant metastasis, mucinous carcinoma, and subsequent outcome after surgery in colorectal cancer [125]. Importantly, STN is involved in colon carcinogenesis and it is detectable in premalignant lesions of colon, and is therefore potentially suited for early detection [126, 127]. Interestingly, the highest levels of SLX is exhibited by three of the most devastating cancers: pancreatic, lung, and gastric cancer [128]. It is reported that especially SLX-containing triantennary N-glycans are increased in the serum of breast, prostate, ovarian, pancreatic, melanoma, peritoneal, and endometrial cancer patients [128]. In line with this, by employing sensitive HPLC-based high-throughput technology, Saldova et al. investigated the glycosylation of serum acute-phase glycoproteins haptoglobin, α-1-acid glycoprotein, and α1-antichymotrypsin which often carry such triantennary N-glycans and contained both elevated levels of SLX as well as core fucosylated agalactosylated diantennary glycans in ovarian cancer patients compared to controls. Furthermore, SLX levels combined with core fucosylated, agalactosylated diantennary glycans could significantly improve the discrimination of benign disease from ovarian cancer and showed a potential use as complementary markers for CA125 in ovarian cancer diagnosis [20]. Balmaña et al. identified that ceruloplasmin, one of the proteins synthetized in the liver, expressed increased SLX levels in the serum of pancreatic cancer patients and suggested the ratio of SLX/ceruloplasmin as useful biomarker for pancreatic cancer [23]. In addition, Tang et al. employed a new method called motif profiling and found that SLX combined with CA19–9 has potential use in the diagnosis of pancreatic cancer [129].
In summary, serum sialylation changes of glyco-antigens containing sialic acids showed great value as cancer biomarkers for improved diagnosis as well as prognosis and patient stratification in many types of cancer. As previously described, however, proper validation studies are required and a combination of glycan-epitope alterations together with other markers such as protein concentrations appears to be the most promising approach for high sensitivity and specificity.
N-glycolylneuraminic acids and sialic acid modifications
Sialic acids present on cells of mammals are primarily composed of Neu5Ac and Neu5Gc, the two prevalent derivatives of sialic acids. As previously mentioned, humans are unable to synthesize Neu5Gc [26, 27]. Despite this fact, Neu5Gc was found on human epithelial and endothelial cell surfaces as a result of incorporation from dietary sources [130]. As a foreign antigen, Neu5Gc has been reported to induce an immune response which can stimulate chronic inflammation [131]. Neu5Gc is also associated with cancer pathologies and elevated levels have been detected in various types of cancer such as colorectal cancer [132, 133], liver cancer [133, 134] and ovarian cancer [135]. Furthermore, incorporated Neu5Gc can be targeted by anti-Neu5Gc antibodies in the circulation which have been identified as potential serum cancer biomarkers in humans [136]. Neu5Gc incorporation from dietary sources and its interaction with the anti-Neu5Gc antibodies in the serum have been implicated in tumor-promoting inflammation which is one of the hallmarks of cancer [137, 138]. Recently, Neu5Gc-glycoconjugates have been investigated as promising cancer vaccines, especially targeting sialylated glycolipids [139,140,141,142].
Furthermore, O-acetylation is a common modification of sialic acids. Though some studies have investigated their role in cancer tissues and cells lines [143, 144], to our knowledge, nothing is known on sialic acid O-acetylation of glycoconjugates in serum and this needs further investigation.
Utility of serum sialidase and sialyltransferase levels in cancer diagnosis, progression and prognosis
It has been reported that changes in the expression and activity of sialyltransferases are important switches in the sialic acid metabolism of a variety of malignant cells [145]. Abnormal levels of several glycosyltransferases have been reported to be implicated in human cancer [78, 146,147,148,149,150,151,152]. Sialyltransferases were found to be elevated in the serum obtained from both animals and humans which bear metastasizing tumors (primary sites of the origin include: bile duct, colon, stomach, pancreas, lung, skin, breast) [147, 153,154,155]. Total serum sialyltransferase levels, independent of the type, were systematically studied in a long-term follow-up (measurement of the enzyme levels at 3-month intervals after surgery between 8 and 36 months) in 135 breast cancer patients, using desialylated fetuin as an acceptor to measure the enzyme activity. Results showed that sialyltransferase activities increased with breast cancer stage, indicating that serial measurements of these enzymes could be a reliable marker for monitoring disease activity and the success or failure of therapy [156]. Raval et al. investigated the alterations of sialyltransferase activity, but also levels of sialic acids and sialoproteins in breast cancer. This study found that elevation of all three serum markers was positively related to tumor presence and negatively associated with response to anti-cancer treatment, making high levels of these markers at diagnosis an indicator of poor prognosis [157]. Similar observations were also made for oral cavity cancer by the same group of Raval et al. [158]. In accordance with this, Shah et al. reported that serum α2–6-sialyltransferase levels have potential utility in early detection, prognosis and treatment monitoring of oral cancer [50]. These studies suggested the potential utility of these sialylation-related markers including sialyltransferases in cancer detection, prognosis and evaluation of clinical outcome. In contrast, Silver et al. reported that sialyltransferase measurement is relatively insensitive and has limited use in cancer prognosis [75].
Contrary to glycosyltransferases, human sialidases catalyze the removal of sialic acid residues from glycoproteins or glycolipids and have been shown to be involved in cancer progression [159]. It has been reported that sialidases can be detected in human cells and tissues. However, only few studies have focused on their presence in human serum/plasma [160,161,162]. Neuraminidase (NEU)3 has been found to be markedly up-regulated in a variety of human cancers such as colon [163], renal [164], ovarian [165], and prostate [166] cancer. It contributes to the augmentation of malignant properties of cancer cells most likely by causing the disturbance of transmembrane signaling [163], but only a few studies on serum/plasma have been reported. Though for many cancers increased sialylation levels have been reported, Hata et al. found for the first time that NEU3 could be detected in the serum of prostate cancer patients, who showed a significant increase of NEU3 activity in the serum compared with healthy subjects [167]. In their preliminary experiment, they also found higher sialidase activities in the serum of patients with bladder, testis and renal cancer [167]. Increased serum and tissue sialidase activity, measured as the extent of de-sialylation of fetuin or other glycoconjugates, were also found by Sönmez et al. in breast cancer patients compared to controls [168]. Interestingly, levels of sialidase activity in serum and tissue were significantly different between Grade I–II and III breast cancer patients, indicating the potential use of sialidase in serum and tissue as cancer biomarker for evaluating cancer progression and patient stratification [168].
Though the mechanisms and function of glycan-related enzymes is still largely obscure, measuring these enzymes may turn out to be another approach in order to find potential clinical cancer markers, and warrants further in-depth examination.
Useful techniques for the measurements of serum/plasma sialylation changes
Serum/plasma sialylation changes have been established as a potential tumor marker for patients with various cancers. A variety of methods for the detection and estimation of free sialic acids and sialic acids bound to glycoproteins or glycolipids have been performed. The earliest methods tended to be colorimetric/spectrophotometric assays including orcinol methods, resorcinol methods, periodic acid/thiobarbiturate methods, and the periodic acid/methyl-3-benzothiazolone-2-hydrazone method [169,170,171,172,173,174]. For these methods, sialic acids are first released by acid hydrolysis or neuraminidase treatment, then reacted with reagents to form a chromogen which can be extracted and measured based on their spectral absorption. Some groups have also attempted to chemically dissect serum sialic acid into sialolipid and sialoglycoprotein components in order to measure LSA [175]. While colorimetric/spectrophotometric methods are still widely used, simple to handle and relatively straightforward to be used in the clinics, interferences can lead to overestimation of the sialic acid content. Therefore, different modifications of the colorimetric/spectrophotometric protocols as well as various fluorescence assays [176], enzymatic assays [177], and chromatographic methods for sialic acid content determination have been developed in order to pursue higher sensitivity or specificity, and easy and fast procedures.
Chromatographic separation, in particular, allows the successful separation of the sialic acids from interfering compounds. Different chromatographic approaches have been published for the determination of sialic acid content including direct approaches such as Anion-Exchange Chromatography with Pulsed Amperometric Detection (HPAE-PAD) as well as gas chromatography with mass spectrometry (GC-MS), thin-layer chromatography and the highly sensitive high performance liquid chromatographic (HPLC) method [178,179,180,181]. While approaches such as HPAE-PAD measure the sialic acids directly, others utilize an indirect method through derivatization of the sialic acid followed by HPLC and photometric or fluorescence detection. Early developments explored sugar-borate complexes in combination with different ion exchange HPLC separations and photometric detection, allowing the quantification of (N- and O-acetylated) sialic acids without prior extensive purification [181]. One of the most common fluorescence labels for sialic acids is 1,2-diamino-4,5-methylenedioxybenzene dihydrochloride (DMB) which has been applied to detect and quantify different sialic acid variants in combination with HPLC separation and fluorescence detection as well as liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS) [182, 183].
Mass spectrometry as a detection method has become an important technology for glycan analysis. It offers sensitivity, high accuracy, tolerance for the sample impurity, and compatibility with various separation techniques. MALDI-TOF-MS has become a major approach for glycan profiling of human serum (in high-throughput manner) and identifying sialylated glycoproteins. However, the cleavage of the sialic acid moiety by in- and post-source decay can cause biases in the determination of sialylated glycans by MALDI-MS. Many chemical derivatization methods were introduced to stabilize the sialylated glycan during MALDI-MS analysis including permethylation [184], esterification [185, 186], amidation [187], methylamidation [188] and dimethylamidation [189] and have increased the sensitivity of detection and the stability of sialic acids.
Recently, Wang et al. reported on a highly specific and sensitive LC-MS/MS glycomic method which can be used to quantitatively determine the level of free and conjugated forms of sialic acids, namely N-acetylneuraminic acid, N-glycolylneuraminic acid and 2-keto-3-deoxy-D-glycero-D-galacto-nononic acid (KDN). This was applied to human cancers and a subset of matched lymph nodes [190]. This LC-MS/MS method showed higher sensitivity than the HPLC-based DMB method used in their previous studies [191]. Furthermore, selected reaction monitoring (SRM) has become a very popular MS detection mode for LC-MS/MS methods due to its high capability of selection, high sensitivity and specificity - gradually attracting more attention from researchers regarding qualitative and quantitative analysis of sialic acids [192]. Similar studies have been performed by Shi et al. [193] and Capote et al. [194] concerning sialic acid quantitation in human plasma employing LC-MS/MS and good accuracy and a low coefficient of variation were reported. Notably, when highly sensitive methods are applied, there is a risk of cross-contamination by sialic acids through sample-handling (particularly samples that contain very low levels of sialic acids) . It is recommended that measures should be taken to limit this risk of contamination [195].
Putative mechanisms for the serum/plasma sialylation changes
Many malignant cells are characterized by increased expression of sialic acids on the cell surfaces and membranes [34]. In certain cancers, increased activity of sialyltransferase and the high turnover of tumor cells might lead to spontaneous shedding of aberrant sialic acid-containing cell surface glycoconjugates into the circulation and cause the high sialic acid concentration in the serum [196]. In one study, tumor tissue TSA/TP was found to be significantly higher than serum TSA /TP supporting the hypothesis of an enhancement in tumor sialoglycoconjugate biosynthesis and shedding being the cause of an increase in serum sialic acid content [197]. A decrease in serum sialoglycoconjugate degradation and/or an altered clearing of these glycoconjugates by the liver could also account for the elevation of serum sialic acids [63]. The increased serum sialic acid concentration was directly correlated to an increase in the concentration and the degree of sialylation of tumor secreted products such as alkaline phosphatase, MUC5AC mucin and CA19–9 which are sialoglycoconjugates, as reported by Wongkham et al. [197]. It is speculated that serum MU5AC mucin and antigen CA19–9 are related to the increased amount of white blood cells as well as secretion of cytokines and glycoproteins from immune cells in serum of cancer patients; one possible explanation for the elevation of serum TSA [197]. On the other hand, increased activity of serum sialyltransferases has been shown in several cancers [63, 198, 199] which may reflect an inflammation reaction to the tumor, resulting in more sialic acid-rich glycoprotein synthesis and release from liver [200].
Altered glycosylation regulated by epigenetics such as histone modification, DNA methylation or remodeling of microRNA may be another concept for inducing increased sialylation in cancer which would suggest a systematic transformation from healthy to diseased conditions [201]. For example, increased levels of branching and sialylation of N-glycans after 5-AZA-2′-deoxycytidine treatment in the ovarian cancer cell line OVCAR3 and increased production of the SLX epitope on the MUC1 protein in HCT15 colon cancer cells were found by Saldova et al. [202] and Chachadi et al. [203], respectively.
In addition, higher serum TSA may be related to different concentrations and glycosylation patterns of acute-phase proteins. Accordingly, Crook et al. found significant correlation between serum TSA and serum levels of some acute phase proteins such as α1-antichymotrypsin, α-1-acid glycoprotein and serum C-reactive protein [204]. Taniuchi et al. reported that serum TSA correlates well with the acute-phase protein response and in particular with serum concentrations of α-1-acid glycoprotein [205]. Being an acute phase protein, α-1-acid glycoprotein is synthesized by the liver and secreted into the circulation. Its serum concentration rises in response to inflammatory stimuli, potentially increasing the concentration two- to four-fold [206]. During the early stages of an acute-phase immune response, the SLX levels of α-1-acid glycoprotein increase significantly, which continues throughout the whole acute phase immune response [206,207,208].
The involvement of acute-phase proteins in these clinical conditions forms the most plausible explanation for the elevated serum TSA levels, raising the question of the relationship between cancer and inflammation. On the other hand, studies focusing on liver dysfunction showed a decreased serum level of mucoproteins in liver cirrhosis, which was in opposition to the results in neoplastic transformation [200]. In chronic liver diseases the mean sialic acid level was lower than in a group of non-inflammatory and non-neoplastic diseases [200]. The α-1-acid glycoprotein is markedly affected by a disturbance of synthesis of serum glycoproteins due to chronic liver insufficiency as the liver is the main site of synthesis for these glycoproteins [200]. Another study confirmed the conclusions showing that in cases of liver cirrhosis the TSA concentration is near normal [209]. The explanation is not so clear but may reflect heterogeneity of levels of acute phase proteins or differing degrees of glycoprotein sialylation in these individuals. With regard to the aforementioned different types of cancer one would anticipate an acute phase response, though this does not account for the entire serum sialic acid elevation since sialylated glycolipids, so-called gangliosides, also contribute slightly to the serum TSA content (1–2%) [210].
The potential mechanisms underlying the sialylation-related changes in cancer discussed here also illustrated the complexity and limitations in using altered sialic acids as tumor markers as it has an acute phase reactant and most of the proteins that increase in acute phase are sialylated (dominated by α2–3-sialylation on these proteins in the form of SLX). Therefore, most investigators indicated that serum sialic acids could be more useful in monitoring of cancer patients and follow up after therapy rather than early-detection.
Conclusion and future perspectives
Serum/plasma sialylation changes in cancer patients have been studied to evaluate their potential as tumor marker. Sialylation changes in serum/plasma can be detected during cancer initiation, progression and treatment which show potential in a variety of clinical applications. Summarizing the applications of serum sialylation-related markers discussed above, the majority of cancers showed alterations in total sialic acid levels, concentrations and degrees of sialylation of specific glycoproteins, levels of specific sialo-glycan antigens and/or activity of sialylation-related enzymes, i.e. sialidases and sialyltransferases (see also Tables 1, 2, 3, 4 for a comprehensive overview). Various investigators have suggested that overall serum sialic acid concentration may be a valuable biochemical marker in detecting metastases, stages of a disease, risk for recurrence and evaluating therapeutic response, potentially in combination with other markers to increase the cancer type-specificity. Sialidases, which catalyze the removal of sialic acid residues from glycoproteins and glycolipids, and sialyltransferases, which are involved in the formation of sialylated glycans, were also found to be differentially expressed in the serum, providing likewise potential cancer biomarkers, but studies hitherto have displayed some limitations. Particularly worth mentioning are alterations in sialylation of individual glycoproteins which can help to improve the specificity of sialylation-related markers. For example, increased α2–3-linked sialylation of PSA is reported to have potential as biomarker for prostate cancer. Additionally, increased IgG sialylation is useful for the assessment of risk of multiple myeloma, while decreased IgG sialylation is associated with poor prognosis in colorectal cancer. Furthermore, sialylation changes on acute-phase proteins as well as alterations of glyco-antigens such as CA19–9 and SLX in serum showed value for diagnosis as well as prognosis and patient stratification.
As serum/plasma sialylation changes have been established as a potential tumor marker for patients with various cancers, more accurate methods with high sensitivity, high specificity and less time-consuming sample preparations are needed for the detection and evaluation of free and glycosidically-bound sialic acids. A variety of reports on the improvements of the methods have been published and mass spectrometry-based methods are gaining more interest.
Moreover, the mechanism behind the sialylation-related changes in different cancers remain poorly understood, though several possibilities of the increase in serum sialic acid are currently being considered: an intensified release of sialic acid-containing cell surface glycoconjugates from tumor cells, an increased concentration and/or glycosylation of normal serum glycoproteins, secondary inflammatory reactions leading to an output of acute phase proteins from the liver, or increased sialylation of serum glycoproteins resulting from epigenetic regulation.
Overall, serum sialylation changes are prominent in various cancers and offer a broad range of opportunities for novel markers. Future research should focus on defining these sialylation changes in a more specific manner revealing protein-specific changes and, importantly, validation studies with larger sample cohorts are needed to confirm and eliminate current markers. Likewise, mechanistic insights are needed in order to shed light on the potential interplay of glycomic changes in the malignant tissue, the liver and plasma cells, and the role of sialylation changes inflicted post-secretion by sialidases and transferases.
References
Jemal, A., Bray, F., Center, M.M., Ferlay, J., Ward, E., Forman, D.: Global cancer statistics. CA: a cancer journal for clinicians 61(2), 69–90 (2011). doi:https://doi.org/10.3322/caac.20107
Torre, L.A., Bray, F., Siegel, R.L., Ferlay, J., Lortet-Tieulent, J., Jemal, A.: Global cancer statistics, 2012. CA: a cancer journal for clinicians 65(2), 87–108 (2015). doi:https://doi.org/10.3322/caac.21262
Mishra, A., Verma, M.: Cancer biomarkers: are we ready for the prime time? Cancers 2(1), 190–208 (2010). doi:https://doi.org/10.3390/cancers2010190
Mabert, K., Cojoc, M., Peitzsch, C., Kurth, I., Souchelnytskyi, S., Dubrovska, A.: Cancer biomarker discovery: current status and future perspectives. International journal of radiation biology 90(8), 659–677 (2014). doi:https://doi.org/10.3109/09553002.2014.892229
Almeida, A., Kolarich, D.: The promise of protein glycosylation for personalised medicine. Biochimica et biophysica acta 1860(8), 1583–1595 (2016). doi:https://doi.org/10.1016/j.bbagen.2016.03.012
Adamczyk, B., Tharmalingam, T., Rudd, P.M.: Glycans as cancer biomarkers. Biochimica et biophysica acta 1820(9), 1347–1353 (2012). doi:https://doi.org/10.1016/j.bbagen.2011.12.001
Glinsky, G.V.: Antigen presentation, aberrant glycosylation and tumor progression. Critical reviews in oncology/hematology. 17(1), 27–51 (1994)
Ohtsubo, K., Marth, J.D.: Glycosylation in cellular mechanisms of health and disease. Cell 126(5), 855–867 (2006). doi:https://doi.org/10.1016/j.cell.2006.08.019
Vajaria, B.N., Patel, P.S.: Glycosylation: a hallmark of cancer? Glycoconjugate journal 34(2), 147–156 (2017). doi:https://doi.org/10.1007/s10719-016-9755-2
Reis, C.A., Osorio, H., Silva, L., Gomes, C., David, L.: Alterations in glycosylation as biomarkers for cancer detection. Journal of clinical pathology 63(4), 322–329 (2010). doi:https://doi.org/10.1136/jcp.2009.071035
Helenius, A., Aebi, M.: Intracellular functions of N-linked glycans. Science 291(5512), 2364–2369 (2001). doi:https://doi.org/10.1126/science.291.5512.2364
Rudd, P.M., Woods, R.J., Wormald, M.R., Opdenakker, G., Downing, A.K., Campbell, I.D., Dwek, R.A.: The effects of variable glycosylation on the functional activities of ribonuclease, plasminogen and tissue plasminogen activator. Biochimica et biophysica acta. 1248(1), 1–10 (1995)
Rudd, P.M., Wormald, M.R., Stanfield, R.L., Huang, M., Mattsson, N., Speir, J.A., DiGennaro, J.A., Fetrow, J.S., Dwek, R.A., Wilson, I.A.: Roles for glycosylation of cell surface receptors involved in cellular immune recognition. Journal of molecular biology 293(2), 351–366 (1999). doi:https://doi.org/10.1006/jmbi.1999.3104
Brockhausen, I.: Pathways of O-glycan biosynthesis in cancer cells. Biochimica et biophysica acta. 1473(1), 67–95 (1999)
Schwarz, F., Aebi, M.: Mechanisms and principles of N-linked protein glycosylation. Current opinion in structural biology 21(5), 576–582 (2011). doi:https://doi.org/10.1016/j.sbi.2011.08.005
Clerc, F., Reiding, K.R., Jansen, B.C., Kammeijer, G.S., Bondt, A., Wuhrer, M.: Human plasma protein N-glycosylation. Glycoconjugate journal 33(3), 309–343 (2016). doi:https://doi.org/10.1007/s10719-015-9626-2
Drake, P.M., Cho, W., Li, B., Prakobphol, A., Johansen, E., Anderson, N.L., Regnier, F.E., Gibson, B.W., Fisher, S.J.: Sweetening the pot: adding glycosylation to the biomarker discovery equation. Clinical chemistry 56(2), 223–236 (2010). doi:https://doi.org/10.1373/clinchem.2009.136333
An, H.J., Kronewitter, S.R., de Leoz, M.L., Lebrilla, C.B.: Glycomics and disease markers. Current opinion in chemical biology 13(5–6), 601–607 (2009). doi:https://doi.org/10.1016/j.cbpa.2009.08.015
Takahashi, S., Sugiyama, T., Shimomura, M., Kamada, Y., Fujita, K., Nonomura, N., Miyoshi, E., Nakano, M.: Site-specific and linkage analyses of fucosylated N-glycans on haptoglobin in sera of patients with various types of cancer: possible implication for the differential diagnosis of cancer. Glycoconjugate journal 33(3), 471–482 (2016). doi:https://doi.org/10.1007/s10719-016-9653-7
Saldova, R., Royle, L., Radcliffe, C.M., Abd Hamid, U.M., Evans, R., Arnold, J.N., Banks, R.E., Hutson, R., Harvey, D.J., Antrobus, R., Petrescu, S.M., Dwek, R.A., Rudd, P.M.: Ovarian cancer is associated with changes in glycosylation in both acute-phase proteins and IgG. Glycobiology 17(12), 1344–1356 (2007). doi:https://doi.org/10.1093/glycob/cwm100
Arnold, J.N., Saldova, R., Galligan, M.C., Murphy, T.B., Mimura-Kimura, Y., Telford, J.E., Godwin, A.K., Rudd, P.M.: Novel glycan biomarkers for the detection of lung cancer. Journal of proteome research 10(4), 1755–1764 (2011). doi:https://doi.org/10.1021/pr101034t
Bones, J., Byrne, J.C., O’Donoghue, N., McManus, C., Scaife, C., Boissin, H., Nastase, A., Rudd, P.M.: Glycomic and glycoproteomic analysis of serum from patients with stomach cancer reveals potential markers arising from host defense response mechanisms. Journal of proteome research 10(3), 1246–1265 (2011). doi:https://doi.org/10.1021/pr101036b
Balmana, M., Sarrats, A., Llop, E., Barrabes, S., Saldova, R., Ferri, M.J., Figueras, J., Fort, E., de Llorens, R., Rudd, P.M., Peracaula, R.: Identification of potential pancreatic cancer serum markers: Increased sialyl-Lewis X on ceruloplasmin. Clinica chimica acta; international journal of clinical chemistry 442, 56–62 (2015). doi:https://doi.org/10.1016/j.cca.2015.01.007
Ledeen, R.W.Y.: R K: Chemistry and analysis of sialic acid. Springer, Biological roles of sialic acid (1976)
Schauer, R.: Sialic Acids and Their Role as Biological Masks. Trends Biochem Sci 10(9), 357–360 (1985). doi:https://doi.org/10.1016/0968-0004(85)90112-4
Muchmore, E.A., Diaz, S., Varki, A.: A structural difference between the cell surfaces of humans and the great apes. American journal of physical anthropology 107(2), 187–198 (1998). doi:https://doi.org/10.1002/(SICI)1096-8644(199810)107:2 < 187::AID-AJPA5 > 3.0.CO;2-S.
Irie, A., Koyama, S., Kozutsumi, Y., Kawasaki, T., Suzuki, A.: The molecular basis for the absence of N-glycolylneuraminic acid in humans. The Journal of biological chemistry. 273(25), 15,866–15,871 (1998)
Schauer, R.: Cell Biology Monograph, vol. 10. Sialic Acids Chemistry, Metabolism and Function. Springer Verlag Vienna (1982)
Jeanloz, R.W.C.: John F: The biological role of sialic acid at the surface of the cell. Springer, Biological roles of sialic acid (1976)
Uslu, C., Taysi, S., Akcay, F., Sutbeyaz, M.Y., Bakan, N.: Serum free and bound sialic acid and alpha-1-acid glycoprotein in patients with laryngeal cancer. Annals of clinical and laboratory science. 33(2), 156–159 (2003)
Narayanan, S.: Sialic acid as a tumor marker. Annals of clinical and laboratory science. 24(4), 376–384 (1994)
Schauer, R.: Achievements and challenges of sialic acid research. Glycoconjugate journal 17(7–9), 485–499 (2000).
Shen, L., Luo, Z., Wu, J., Qiu, L., Luo, M., Ke, Q., Dong, X.: Enhanced expression of alpha2,3-linked sialic acids promotes gastric cancer cell metastasis and correlates with poor prognosis. International journal of oncology 50(4), 1201–1210 (2017). doi:https://doi.org/10.3892/ijo.2017.3882
Bull, C., Stoel, M.A., den Brok, M.H., Adema, G.J.: Sialic acids sweeten a tumor’s life. Cancer research 74(12), 3199–3204 (2014). doi:https://doi.org/10.1158/0008-5472.CAN-14-0728
Bull, C., den Brok, M.H., Adema, G.J.: Sweet escape: sialic acids in tumor immune evasion. Biochimica et biophysica acta 1846(1), 238–246 (2014). doi:https://doi.org/10.1016/j.bbcan.2014.07.005
Saldova, R., Piccard, H., Perez-Garay, M., Harvey, D.J., Struwe, W.B., Galligan, M.C., Berghmans, N., Madden, S.F., Peracaula, R., Opdenakker, G., Rudd, P.M.: Increase in sialylation and branching in the mouse serum N-glycome correlates with inflammation and ovarian tumour progression. PloS one. 8(8), e71159 (2013). https://doi.org/10.1371/journal.pone.0071159
Scherrmacher, V., Altevogt, P., Fogel, M.: Importance of cell surface carbohydrates in cancer cell adhesion, invasion and metastasis, vol. 2. In: Does sialic acid direct metastatic behavior? (1982)
Zhao, Y., Li, Y., Ma, H., Dong, W., Zhou, H., Song, X., Zhang, J., Jia, L.: Modification of sialylation mediates the invasive properties and chemosensitivity of human hepatocellular carcinoma. Molecular & cellular proteomics: MCP 13(2), 520–536 (2014). doi:https://doi.org/10.1074/mcp.M113.034025
Zhao, Y., Wei, A., Zhang, H., Chen, X., Wang, L., Zhang, H., Yu, X., Yuan, Q., Zhang, J.: Wang, S.: alpha2,6-Sialylation mediates hepatocellular carcinoma growth in vitro and in vivo by targeting the Wnt/beta-catenin pathway. Oncogenesis. 6(5), e343 (2017). https://doi.org/10.1038/oncsis.2017.40
Vajaria, B.N., Patel, K.R., Begum, R., Patel, P.S.: Sialylation: an Avenue to Target Cancer Cells. Pathology oncology research: POR. 22(3), 443–447 (2016). https://doi.org/10.1007/s12253-015-0033-6
Veillon, L., Fakih, C., Abou-El-Hassan, H., Kobeissy, F., Mechref, Y.: Glycosylation Changes in Brain Cancer. ACS chemical neuroscience 9(1), 51–72 (2018). doi:https://doi.org/10.1021/acschemneuro.7b00271
Borzym-Kluczyk, M., Radziejewska, I., Cechowska-Pasko, M., Darewicz, B.: Reduced expression of E-cadherin and increased sialylation level in clear cell renal cell carcinoma. Acta biochimica Polonica 64(3), 465–470 (2017). doi:https://doi.org/10.18388/abp.2015_1215
Skipski, V.P., Katopodis, N., Prendergast, J.S., Stock, C.C.: Gangliosides in blood serum of normal rats and Morris hepatoma 5123tc-bearing rats. Biochemical and biophysical research communications. 67(3), 1122–1127 (1975)
Khadapkar, S.V., Sheth, N.A., Bhide, S.V.: Independence of sialic acid levels in normal and malignant growth. Cancer research. 35(6), 1520–1523 (1975)
Nicol, B.M., Prasad, S.B.: Sialic acid changes in Dalton’s lymphoma-bearing mice after cyclophosphamide and cisplatin treatment. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas 35(5), 549–553 (2002).
Winzler, R.J.: Determination of serum glycoproteins. Methods of Biochemical Analysis. 2, 279–311 (1955)
Macbeth, R.A.L., Bekesi, J.G.: Plasma Glycoproteins in Various Disease States Including Carcinoma. Cancer research. 22(10), 1170–1176 (1962)
Brozmanova, E., Skrovina, B.: Sialic-Acid and Bone Tumors. Neoplasma 19(2), 115 − + (1972).
Schutter, E.M., Visser, J.J., van Kamp, G.J., Mensdorff-Pouilly, S., van Dijk, W., Hilgers, J., Kenemans, P.: The utility of lipid-associated sialic acid (LASA or LSA) as a serum marker for malignancy. A review of the literature. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine 13(3), 121–132 (1992).
Shah, M.H., Telang, S.D., Shah, P.M., Patel, P.S.: Tissue and serum alpha 2–3- and alpha 2–6-linkage specific sialylation changes in oral carcinogenesis. Glycoconjugate journal 25(3), 279–290 (2008). doi:https://doi.org/10.1007/s10719-007-9086-4
Sawhney, H., Kumar, C.A.: Correlation of serum biomarkers (TSA & LSA) and epithelial dysplasia in early diagnosis of oral precancer and oral cancer. Cancer biomarkers: section A of Disease markers 10(1), 43–49 (2011). doi:https://doi.org/10.3233/CBM-2012-0226
Joshi, M., Patil, R.: Estimation and comparative study of serum total sialic acid levels as tumor markers in oral cancer and precancer. Journal of cancer research and therapeutics. 6(3), 263–266 (2010). https://doi.org/10.4103/0973-1482.73339
Patolia, K., Shah, M.: An Assessment of Serum Total Sialic Acid in Oral Leukoplakia and Oral Squamous Cell Carcinoma at Nadiad, Gujarat. International Journal of Medical Paediatrics and Oncology 2(2), 56–59.
Bhanushree, R.: Clinical Relevance of Serum Total Sialic Acid In Oral Leukoplakia and Oral Squamous Cell Carcinoma-A Randomized Study. Global Journal For Research Analysis 4(12) (2016).
Vajaria, B.N., Patel, K.R., Begum, R., Shah, F.D., Patel, J.B., Shukla, S.N., Patel, P.S.: Evaluation of serum and salivary total sialic acid and alpha-l-fucosidase in patients with oral precancerous conditions and oral cancer. Oral surgery, oral medicine, oral pathology and oral radiology 115(6), 764–771 (2013). doi:https://doi.org/10.1016/j.oooo.2013.01.004
Rajpura, K.B., Patel, P.S., Chawda, J.G., Shah, R.M.: Clinical significance of total and lipid bound sialic acid levels in oral pre-cancerous conditions and oral cancer. Journal of oral pathology & medicine: official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology 34(5), 263–267 (2005). doi:https://doi.org/10.1111/j.1600-0714.2004.00210.x
Krishnan, K., Balasundaram, S.: Evaluation of Total and Lipid Bound Sialic Acid in Serum in Oral Leukoplakia. Journal of clinical and diagnostic research: JCDR. 11(3), ZC25–ZC27 (2017). https://doi.org/10.7860/JCDR/2017/16483.9497
Berbec, H., Paszkowska, A., Siwek, B., Gradziel, K., Cybulski, M.: Total serum sialic acid concentration as a supporting marker of malignancy in ovarian neoplasia. European journal of gynaecological oncology 20(5–6), 389–392 (1999).
Wongkham, S., Boonla, C., Kongkham, S., Wongkham, C., Bhudhisawasdi, V., Sripa, B.: Serum total sialic acid in cholangiocarcinoma patients: an ROC curve analysis. Clinical biochemistry. 34(7), 537–541 (2001)
Patel, P.S., Rawal, G.N., Balar, D.B.: Importance of serum sialic acid and lactate dehydrogenase in diagnosis and treatment monitoring of cervical cancer patients. Gynecologic oncology 50(3), 294–299 (1993). doi:https://doi.org/10.1006/gyno.1993.1214
Patel, P.S., Adhvaryu, S.G., Balar, D.B., Parikh, B.J., Shah, P.M.: Clinical application of serum levels of sialic acid, fucose and seromucoid fraction as tumour markers in human leukemias. Anticancer research. 14(2B), 747–751 (1994)
Sebzda, T., Saleh, Y., Gburek, J., Warwas, M., Andrzejak, R., Siewinski, M., Rudnicki, J.: Total and lipid-bound plasma sialic acid as diagnostic markers in colorectal cancer patients: correlation with cathepsin B expression in progression to Dukes stage. Journal of experimental therapeutics & oncology 5(3), 223–229 (2006).
Feijoo, C., Paez de la Cadena, M., Rodriguez-Berrocal, F.J., Martinez-Zorzano, V.S.: Sialic acid levels in serum and tissue from colorectal cancer patients. Cancer letters 112(2), 155–160 (1997).
Hogan-Ryan, A., Fennelly, J.J., Jones, M., Cantwell, B., Duffy, M.J.: Serum sialic acid and CEA concentrations in human breast cancer. British journal of cancer. 41(4), 587–592 (1980)
Krolikowski, F.J., Reuter, K., Waalkes, T.P., Sieber, S.M., Adamson, R.H.: Serum sialic acid levels in lung cancer patients. Pharmacology. 14(1), 47–51 (1976)
Dwivedi, C., Dixit, M., Hardy, R.E.: Plasma lipid-bound sialic acid alterations in neoplastic diseases. Experientia. 46(1), 91–94 (1990)
Tewarson, S.L., Mittal, V.P., Singh, M., Gupta, G.P.: Serum sialic acid--an important cancer marker. Indian journal of cancer. 30(3), 125–131 (1993)
Cebi, A., Mert, H., Mert, N.: Evaluation of levels of some tumor markers, acute phase proteins, sialic acid and lipid bound sialic acid before and after treatment in stomach cancer patients. Febs J. 275, 395–395 (2008)
Kokoglu, E., Uslu, E., Uslu, I., Hatemi, H.H.: Serum and tissue total sialic acid as a marker for human thyroid cancer. Cancer letters. 46(1), 1–5 (1989)
Erbil, K.M., Jones, J.D., Klee, G.G.: Use and limitations of serum total and lipid-bound sialic acid concentrations as markers for colorectal cancer. Cancer. 55(2), 404–409 (1985)
Verazin, G., Riley, W.M., Gregory, J., Tautu, C., Prorok, J.J., Alhadeff, J.A.: Serum sialic acid and carcinoembryonic levels in the detection and monitoring of colorectal cancer. Diseases of the colon and rectum. 33(2), 139–142 (1990)
Dnistrian, A.M., Schwartz, M.K., Katopodis, N., Fracchia, A.A., Stock, C.C.: Serum lipid-bound sialic acid as a marker in breast cancer. Cancer. 50(9), 1815–1819 (1982)
Ros-Bullon, M.R., Sanchez-Pedreno, P., Martinez-Liarte, J.H.: Serum sialic acid in malignant melanoma patients: an ROC curve analysis. Anticancer research. 19(4C), 3619–3622 (1999)
Silver, H.K., Rangel, D.M., Morton, D.L.: Serum sialic acid elevations in malignant melanoma patients. Cancer. 41(4), 1497–1499 (1978)
Silver, H.K., Karim, K.A., Archibald, E.L., Salinas, F.A.: Serum sialic acid and sialyltransferase as monitors of tumor burden in malignant melanoma patients. Cancer research. 39(12), 5036–5042 (1979)
Plucinsky, M.C., Riley, W.M., Prorok, J.J., Alhadeff, J.A.: Total and lipid-associated serum sialic acid levels in cancer patients with different primary sites and differing degrees of metastatic involvement. Cancer. 58(12), 2680–2685 (1986)
Paszkowska, A., Berbec, H., Semczuk, A., Cybulski, M.: Sialic acid concentration in serum and tissue of endometrial cancer patients. European journal of obstetrics, gynecology, and reproductive biology. 76(2), 211–215 (1998)
Kim, Y.S., Isaacs, R.: Glycoprotein metabolism in inflammatory and neoplastic diseases of the human colon. Cancer research. 35(8), 2092–2097 (1975)
Kim, Y.S., Isaacs, R., Perdomo, J.M.: Alterations of membrane glycopeptides in human colonic adenocarcinoma. Proceedings of the National Academy of Sciences of the United States of America. 71(12), 4869–4873 (1974)
Dall’Olio, F., Malagolini, N., di Stefano, G., Minni, F., Marrano, D., Serafini-Cessi, F.: Increased CMP-NeuAc:Gal beta 1,4GlcNAc-R alpha 2,6 sialyltransferase activity in human colorectal cancer tissues. International journal of cancer 44(3), 434–439 (1989).
Romppanen, J., Eskelinen, M., Tikanoja, S., Mononen, I.: Total and lipid-bound serum sialic acid in benign and malignant breast disease. Anticancer research. 17(2B), 1249–1253 (1997)
Vivas, I., Spagnuolo, L., Palacios, P.: Total and lipid-bound serum sialic acid as markers for carcinoma of the uterine cervix. Gynecologic oncology. 46(2), 157–162 (1992)
Tautu, C., Pee, D., Dunsmore, M., Prorok, J.J., Alhadeff, J.A.: Evaluation of serum sialic acid and carcinoembryonic antigen for the detection of early-stage colorectal cancer. Journal of clinical laboratory analysis. 5(4), 247–254 (1991)
Pinho, S.S., Reis, C.A.: Glycosylation in cancer: mechanisms and clinical implications. Nature reviews. Cancer 15(9), 540–555 (2015). doi:https://doi.org/10.1038/nrc3982
Stowell, S.R., Ju, T., Cummings, R.D.: Protein glycosylation in cancer. Annual review of pathology 10, 473–510 (2015). doi:https://doi.org/10.1146/annurev-pathol-012414-040438
Chambers, A.G., Percy, A.J., Simon, R., Borchers, C.H.: MRM for the verification of cancer biomarker proteins: recent applications to human plasma and serum. Expert review of proteomics 11(2), 137–148 (2014). doi:https://doi.org/10.1586/14789450.2014.877346
Moyer, V.A., Force, U.S.P.S.T.: Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Annals of internal medicine 157(2), 120–134 (2012). doi:https://doi.org/10.7326/0003-4819-157-2-201,207,170-00459
Tajiri, M., Ohyama, C., Wada, Y.: Oligosaccharide profiles of the prostate specific antigen in free and complexed forms from the prostate cancer patient serum and in seminal plasma: a glycopeptide approach. Glycobiology 18(1), 2–8 (2008). doi:https://doi.org/10.1093/glycob/cwm117
Pihikova, D., Kasak, P., Kubanikova, P., Sokol, R., Tkac, J.: Aberrant sialylation of a prostate-specific antigen: Electrochemical label-free glycoprofiling in prostate cancer serum samples. Analytica chimica acta 934, 72–79 (2016). doi:https://doi.org/10.1016/j.aca.2016.06.043
Llop, E., Ferrer-Batalle, M., Barrabes, S., Guerrero, P.E., Ramirez, M., Saldova, R., Rudd, P.M., Aleixandre, R.N., Comet, J., de Llorens, R., Peracaula, R.: Improvement of Prostate Cancer Diagnosis by Detecting PSA Glycosylation-Specific Changes. Theranostics 6(8), 1190–1204 (2016). doi:https://doi.org/10.7150/thno.15226
Yoneyama, T., Ohyama, C., Hatakeyama, S., Narita, S., Habuchi, T., Koie, T., Mori, K., Hidari, K.I., Yamaguchi, M., Suzuki, T., Tobisawa, Y.: Measurement of aberrant glycosylation of prostate specific antigen can improve specificity in early detection of prostate cancer. Biochemical and biophysical research communications 448(4), 390–396 (2014). doi:https://doi.org/10.1016/j.bbrc.2014.04.107
Kirwan, A., Utratna, M., O’Dwyer, M.E., Joshi, L., Kilcoyne, M.: Glycosylation-Based Serum Biomarkers for Cancer Diagnostics and Prognostics. BioMed research international 2015, 490,531 (2015). doi:https://doi.org/10.1155/2015/490531
Saeland, E., Belo, A.I., Mongera, S., van Die, I., Meijer, G.A., van Kooyk, Y.: Differential glycosylation of MUC1 and CEACAM5 between normal mucosa and tumour tissue of colon cancer patients. International journal of cancer 131(1), 117–128 (2012). doi:https://doi.org/10.1002/ijc.26354
Wu, J., Xie, X., Nie, S., Buckanovich, R.J., Lubman, D.M.: Altered expression of sialylated glycoproteins in ovarian cancer sera using lectin-based ELISA assay and quantitative glycoproteomics analysis. Journal of proteome research 12(7), 3342–3352 (2013). doi:https://doi.org/10.1021/pr400169n
Kontro, H., Joenvaara, S., Haglund, C., Renkonen, R.: Comparison of sialylated N-glycopeptide levels in serum of pancreatic cancer patients, acute pancreatitis patients, and healthy controls. Proteomics 14(15), 1713–1723 (2014). doi:https://doi.org/10.1002/pmic.201300270
Zhao, J., Simeone, D.M., Heidt, D., Anderson, M.A., Lubman, D.M.: Comparative serum glycoproteomics using lectin selected sialic acid glycoproteins with mass spectrometric analysis: application to pancreatic cancer serum. Journal of proteome research 5(7), 1792–1802 (2006). doi:https://doi.org/10.1021/pr060034r
Ferens-Sieczkowska, M., Kratz, E.M., Kossowska, B., Passowicz-Muszynska, E., Jankowska, R.: Comparison of haptoglobin and alpha(1)-acid glycoprotein glycosylation in the sera of small cell and non-small cell lung cancer patients. Postepy higieny i medycyny doswiadczalnej. 67, 828–836 (2013)
Parekh, R.B., Dwek, R.A., Sutton, B.J., Fernandes, D.L., Leung, A., Stanworth, D., Rademacher, T.W., Mizuochi, T., Taniguchi, T., Matsuta, K., et al.: Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 316(6027), 452–457 (1985)
Kodar, K., Stadlmann, J., Klaamas, K., Sergeyev, B., Kurtenkov, O.: Immunoglobulin G Fc N-glycan profiling in patients with gastric cancer by LC-ESI-MS: relation to tumor progression and survival. Glycoconjugate journal 29(1), 57–66 (2012). doi:https://doi.org/10.1007/s10719-011-9364-z
Ren, S., Zhang, Z., Xu, C., Guo, L., Lu, R., Sun, Y., Guo, J., Qin, R., Qin, W., Gu, J.: Distribution of IgG galactosylation as a promising biomarker for cancer screening in multiple cancer types. Cell research 26(8), 963–966 (2016). doi:https://doi.org/10.1038/cr.2016.83
Theodoratou, E., Thaci, K., Agakov, F., Timofeeva, M.N., Stambuk, J., Pucic-Bakovic, M., Vuckovic, F., Orchard, P., Agakova, A., Din, F.V., Brown, E., Rudd, P.M., Farrington, S.M., Dunlop, M.G., Campbell, H., Lauc, G.: Glycosylation of plasma IgG in colorectal cancer prognosis. Scientific reports. 6, 28,098 (2016). https://doi.org/10.1038/srep28098
Vuckovic, F., Theodoratou, E., Thaci, K., Timofeeva, M., Vojta, A., Stambuk, J., Pucic-Bakovic, M., Rudd, P.M., Derek, L., Servis, D., Wennerstrom, A., Farrington, S.M., Perola, M., Aulchenko, Y., Dunlop, M.G., Campbell, H., Lauc, G.: IgG Glycome in Colorectal Cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 22(12), 3078–3086 (2016). https://doi.org/10.1158/1078-0432.CCR-15-1867
Zhang, D., Chen, B., Wang, Y., Xia, P., He, C., Liu, Y., Zhang, R., Zhang, M., Li, Z.: Disease-specific IgG Fc N-glycosylation as personalized biomarkers to differentiate gastric cancer from benign gastric diseases. Scientific reports. 6, 25,957 (2016). https://doi.org/10.1038/srep25957
Saldova, R., Wormald, M.R., Dwek, R.A., Rudd, P.M.: Glycosylation changes on serum glycoproteins in ovarian cancer may contribute to disease pathogenesis. Disease markers 25(4–5), 219–232 (2008).
Fleming, S.C., Smith, S., Knowles, D., Skillen, A., Self, C.H.: Increased sialylation of oligosaccharides on IgG paraproteins--a potential new tumour marker in multiple myeloma. Journal of clinical pathology. 51(11), 825–830 (1998)
Gornik, O., Lauc, G.: Glycosylation of serum proteins in inflammatory diseases. Disease markers 25(4–5), 267–278 (2008).
Gornik, O., Pavic, T., Lauc, G.: Alternative glycosylation modulates function of IgG and other proteins - implications on evolution and disease. Biochimica et biophysica acta 1820(9), 1318–1326 (2012). doi:https://doi.org/10.1016/j.bbagen.2011.12.004
Schwab, I., Nimmerjahn, F.: Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nature reviews. Immunology 13(3), 176–189 (2013). doi:https://doi.org/10.1038/nri3401
Hakomori, S.: Aberrant glycosylation in cancer cell membranes as focused on glycolipids: overview and perspectives. Cancer research. 45(6), 2405–2414 (1985)
Kannagi, R.: Carbohydrate antigen sialyl Lewis a--its pathophysiological significance and induction mechanism in cancer progression. Chang Gung medical journal. 30(3), 189–209 (2007)
Duraker, N., Hot, S., Polat, Y., Hobek, A., Gencler, N., Urhan, N.: CEA, CA 19–9, and CA 125 in the differential diagnosis of benign and malignant pancreatic diseases with or without jaundice. Journal of surgical oncology 95(2), 142–147 (2007). doi:https://doi.org/10.1002/jso.20604
Liao, Q., Zhao, Y.P., Yang, Y.C., Li, L.J., Long, X., Han, S.M.: Combined detection of serum tumor markers for differential diagnosis of solid lesions located at the pancreatic head. Hepatobiliary & pancreatic diseases international: HBPD INT 6(6), 641–645 (2007).
Nakagoe, T., Fukushima, K., Hirota, M., Kusano, H., Ayabe, H., Tomita, M., Kamihira, S.: An immunohistochemical employer monoclonal antibodies against Le(a), sialyl Le(a), Le(x), and sialyl Le(x) antigens in primary colorectal, carcinomas and lymph node and hepatic lesions. Journal of gastroenterology. 29(2), 129–138 (1994)
Altevogt, P., Fogel, M., Cheingsong-Popov, R., Dennis, J., Robinson, P., Schirrmacher, V.: Different patterns of lectin binding and cell surface sialylation detected on related high- and low-metastatic tumor lines. Cancer research. 43(11), 5138–5144 (1983)
Ballehaninna, U.K., Chamberlain, R.S.: The clinical utility of serum CA 19–9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. Journal of gastrointestinal oncology 3(2), 105–119 (2012). doi:https://doi.org/10.3978/j.issn.2078-6891.2011.021
Stojkovic Lalosevic, M., Stankovic, S., Stojkovic, M., Markovic, V., Dimitrijevic, I., Lalosevic, J., Petrovic, J., Brankovic, M., Pavlovic Markovic, A., Krivokapic, Z.: Can preoperative CEA and CA19–9 serum concentrations suggest metastatic disease in colorectal cancer patients? Hellenic journal of nuclear medicine 20(1), 41–45 (2017). doi:https://doi.org/10.1967/s002449910505
Filella, X., Molina, R., Grau, J.J., Pique, J.M., Garcia-Valdecasas, J.C., Astudillo, E., Biete, A., Bordas, J.M., Novell, A., Campo, E., et al.: Prognostic value of CA 19.9 levels in colorectal cancer. Annals of surgery 216(1), 55–59 (1992).
Nakayama, T., Watanabe, M., Teramoto, T., Kitajima, M.: CA19–9 as a predictor of recurrence in patients with colorectal cancer. Journal of surgical oncology 66(4), 238–243 (1997).
Kouri, M., Pyrhonen, S., Kuusela, P.: Elevated CA19–9 as the most significant prognostic factor in advanced colorectal carcinoma. Journal of surgical oncology 49(2), 78–85 (1992).
Ueda, T., Shimada, E., Urakawa, T.: The clinicopathologic features of serum CA 19–9-positive colorectal cancers. Surgery today 24(6), 518–525 (1994).
Imada, T., Rino, Y., Hatori, S., Takahashi, M., Amano, T., Kondo, J., Suda, T.: Sialyl Tn antigen expression is associated with the prognosis of patients with advanced colorectal cancer. Hepato-gastroenterology. 46(25), 208–214 (1999)
Sato, T., Nishimura, G., Nonomura, A., Miwa, K., Miyazaki, I.: Serological studies on CEA, CA 19–9, STn and SLX in colorectal cancer. Hepato-gastroenterology 46(26), 914–919 (1999).
Jiang, C., Liu, S., He, W., Zhang, B., Xia, L.: The Prognostic and Predictive Value of Carbohydrate Antigen 19–9 in Metastatic Colorectal Cancer Patients with First Line Bevacizumab Containing Chemotherapy. Journal of Cancer 8(8), 1410–1416 (2017). doi:https://doi.org/10.7150/jca.18325
Nakagoe, T., Sawai, T., Tsuji, T., Jibiki, M.A., Nanashima, A., Yamaguchi, H., Yasutake, T., Ayabe, H., Arisawa, K., Ishikawa, H.: Predictive factors for preoperative serum levels of sialy Lewis(x), sialyl Lewis(a) and sialyl Tn antigens in gastric cancer patients. Anticancer research. 22(1A), 451–458 (2002)
Nakagoe, T., Sawai, T., Tsuji, T., Jibiki, M., Nanashima, A., Yamaguchi, H., Kurosaki, N., Yasutake, T., Ayabe, H.: Circulating sialyl Lewis(x), sialyl Lewis(a), and sialyl Tn antigens in colorectal cancer patients: multivariate analysis of predictive factors for serum antigen levels. Journal of gastroenterology. 36(3), 166–172 (2001)
Krishn, S.R., Kaur, S., Smith, L.M., Johansson, S.L., Jain, M., Patel, A., Gautam, S.K., Hollingsworth, M.A., Mandel, U., Clausen, H., Lo, W.C., Fan, W.T., Manne, U., Batra, S.K.: Mucins and associated glycan signatures in colon adenoma-carcinoma sequence: Prospective pathological implication(s) for early diagnosis of colon cancer. Cancer letters 374(2), 304–314 (2016). doi:https://doi.org/10.1016/j.canlet.2016.02.016
Itzkowitz, S.H., Young, E., Dubois, D., Harpaz, N., Bodian, C., Chen, A., Sachar, D.B.: Sialosyl-Tn antigen is prevalent and precedes dysplasia in ulcerative colitis: a retrospective case-control study. Gastroenterology. 110(3), 694–704 (1996)
Lan, Y., Hao, C., Zeng, X., He, Y., Zeng, P., Guo, Z., Zhang, L.: Serum glycoprotein-derived N- and O-linked glycans as cancer biomarkers. American journal of cancer research. 6(11), 2390–2415 (2016)
Tang, H., Singh, S., Partyka, K., Kletter, D., Hsueh, P., Yadav, J., Ensink, E., Bern, M., Hostetter, G., Hartman, D., Huang, Y., Brand, R.E., Haab, B.B.: Glycan motif profiling reveals plasma sialyl-lewis x elevations in pancreatic cancers that are negative for sialyl-lewis A. Molecular & cellular proteomics: MCP 14(5), 1323–1333 (2015). doi:https://doi.org/10.1074/mcp.M114.047837
Alisson-Silva, F., Kawanishi, K., Varki, A.: Human risk of diseases associated with red meat intake: Analysis of current theories and proposed role for metabolic incorporation of a non-human sialic acid. Molecular aspects of medicine. 51, 16–30 (2016). https://doi.org/10.1016/j.mam.2016.07.002
Okerblom, J., Varki, A.: Biochemical, Cellular, Physiological, and Pathological Consequences of Human Loss of N-Glycolylneuraminic Acid. Chembiochem: a European journal of chemical biology. 18(13), 1155–1171 (2017). https://doi.org/10.1002/cbic.201700077
Higashi, H., Hirabayashi, Y., Fukui, Y., Naiki, M., Matsumoto, M., Ueda, S., Kato, S.: Characterization of N-glycolylneuraminic acid-containing gangliosides as tumor-associated Hanganutziu-Deicher antigen in human colon cancer. Cancer research. 45(8), 3796–3802 (1985)
Kawai, T., Kato, A., Higashi, H., Kato, S., Naiki, M.: Quantitative determination of N-glycolylneuraminic acid expression in human cancerous tissues and avian lymphoma cell lines as a tumor-associated sialic acid by gas chromatography-mass spectrometry. Cancer research. 51(4), 1242–1246 (1991)
Koda, T., Aosasa, M., Asaoka, H., Nakaba, H., Matsuda, H.: Application of tyramide signal amplification for detection of N-glycolylneuraminic acid in human hepatocellular carcinoma. International journal of clinical oncology 8(5), 317–321 (2003). doi:https://doi.org/10.1007/s10147-003-0346-4
Diaz, S.L., Padler-Karavani, V., Ghaderi, D., Hurtado-Ziola, N., Yu, H., Chen, X.: Brinkman-Van der Linden. E.C., Varki, A., Varki, N.M.: Sensitive and specific detection of the non-human sialic Acid N-glycolylneuraminic acid in human tissues and biotherapeutic products. PloS one. 4(1), e4241 (2009). https://doi.org/10.1371/journal.pone.0004241
Padler-Karavani, V., Hurtado-Ziola, N., Pu, M., Yu, H., Huang, S., Muthana, S., Chokhawala, H.A., Cao, H., Secrest, P., Friedmann-Morvinski, D., Singer, O., Ghaderi, D., Verma, I.M., Liu, Y.T., Messer, K., Chen, X., Varki, A., Schwab, R.: Human xeno-autoantibodies against a non-human sialic acid serve as novel serum biomarkers and immunotherapeutics in cancer. Cancer research 71(9), 3352–3363 (2011). doi:https://doi.org/10.1158/0008-5472.CAN-10-4102
Pearce, O.M., Laubli, H., Verhagen, A., Secrest, P., Zhang, J., Varki, N.M., Crocker, P.R., Bui, J.D., Varki, A.: Inverse hormesis of cancer growth mediated by narrow ranges of tumor-directed antibodies. Proceedings of the National Academy of Sciences of the United States of America. 111(16), 5998–6003 (2014). https://doi.org/10.1073/pnas.1209067111
Samraj, A.N., Pearce, O.M., Laubli, H., Crittenden, A.N., Bergfeld, A.K., Banda, K., Gregg, C.J., Bingman, A.E., Secrest, P., Diaz, S.L., Varki, N.M., Varki, A.: A red meat-derived glycan promotes inflammation and cancer progression. Proceedings of the National Academy of Sciences of the United States of America. 112(2), 542–547 (2015). https://doi.org/10.1073/pnas.1417508112
Durrant, L.G., Noble, P., Spendlove, I.: Immunology in the clinic review series; focus on cancer: glycolipids as targets for tumour immunotherapy. Clinical and experimental immunology 167(2), 206–215 (2012). doi:https://doi.org/10.1111/j.1365-2249.2011.04516.x
Fernandez, L.E., Gabri, M.R., Guthmann, M.D., Gomez, R.E., Gold, S., Fainboim, L., Gomez, D.E., Alonso, D.F.: NGcGM3 ganglioside: a privileged target for cancer vaccines. Clinical & developmental immunology 2010, 814397 (2010). doi:https://doi.org/10.1155/2010/814397
Fuentes, D., Avellanet, J., Garcia, A., Iglesias, N., Gabri, M.R., Alonso, D.F., Vazquez, A.M., Perez, R., Montero, E.: Combined therapeutic effect of a monoclonal anti-idiotype tumor vaccine against NeuGc-containing gangliosides with chemotherapy in a breast carcinoma model. Breast cancer research and treatment 120(2), 379–389 (2010). doi:https://doi.org/10.1007/s10549-009-0399-9
Hernandez, A.M., Rodriguez, N., Gonzalez, J.E., Reyes, E., Rondon, T., Grinan, T., Macias, A., Alfonso, S., Vazquez, A.M., Perez, R.: Anti-NeuGcGM3 antibodies, actively elicited by idiotypic vaccination in nonsmall cell lung cancer patients, induce tumor cell death by an oncosis-like mechanism. Journal of immunology 186(6), 3735–3744 (2011). doi:https://doi.org/10.4049/jimmunol.1000609
Schauer, R., Schmid, H., Pommerencke, J.R., Iwersen, M., Kohla, G.: Metabolism and role of O-acetylated sialic acids. The Molecular Immunology of Complex Carbohydrates-2. In: Springer (2001)
Mandal, C., Schwartz-Albiez, R., Vlasak, R.: Functions and biosynthesis of O-acetylated sialic acids. Springer, SialoGlyco Chemistry and Biology I (2012)
Perez-Garay, M., Arteta, B., Pages, L., de Llorens, R., de Bolos, C., Vidal-Vanaclocha, F.: Peracaula, R.: alpha2,3-sialyltransferase ST3Gal III modulates pancreatic cancer cell motility and adhesion in vitro and enhances its metastatic potential in vivo. In: PloS one 5(9) (2010). https://doi.org/10.1371/journal.pone.0012524
LaMont, J.T., Isselbacher, K.J.: Alterations in glycosyltransferase activity in human colon cancer. Journal of the National Cancer Institute. 54(1), 53–56 (1975)
Henderson, M., Kessel, D.: Alterations in plasma sialyltransferase levels in patients with neoplastic disease. Cancer. 39(3), 1129–1134 (1977)
Vazquez-Martin, C., Cuevas, E., Gil-Martin, E., Fernandez-Briera, A.: Correlation analysis between tumor-associated antigen sialyl-Tn expression and ST6GalNAc I activity in human colon adenocarcinoma. Oncology 67(2), 159–165 (2004). doi:https://doi.org/10.1159/000081003
Tamura, F., Sato, Y., Hirakawa, M., Yoshida, M., Ono, M., Osuga, T., Okagawa, Y., Uemura, N., Arihara, Y., Murase, K., Kawano, Y., Iyama, S., Takada, K., Hayashi, T., Sato, T., Miyanishi, K., Kobune, M., Takimoto, R., Kato, J.: RNAi-mediated gene silencing of ST6GalNAc I suppresses the metastatic potential in gastric cancer cells. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 19(1), 85–97 (2016). https://doi.org/10.1007/s10120-014-0454-z
Cui, H.X., Wang, H., Wang, Y., Song, J., Tian, H., Xia, C., Shen, Y.: ST3Gal III modulates breast cancer cell adhesion and invasion by altering the expression of invasion-related molecules. Oncology reports 36(6), 3317–3324 (2016). doi:https://doi.org/10.3892/or.2016.5180
Lu, J., Isaji, T., Im, S., Fukuda, T., Kameyama, A., Gu, J.: Expression of N-Acetylglucosaminyltransferase III Suppresses alpha2,3-Sialylation, and Its Distinctive Functions in Cell Migration Are Attributed to alpha2,6-Sialylation Levels. The Journal of biological chemistry. 291(11), 5708–5720 (2016). https://doi.org/10.1074/jbc.M115.712836
Suzuki, O., Abe, M., Hashimoto, Y.: Sialylation by beta-galactoside alpha-2,6-sialyltransferase and N-glycans regulate cell adhesion and invasion in human anaplastic large cell lymphoma. International journal of oncology 46(3), 973–980 (2015). doi:https://doi.org/10.3892/ijo.2015.2818
Alhadeff, J.A., Holzinger, R.T.: Sialyltransferase, sialic acid and sialoglycoconjugates in metastatic tumor and human liver tissue. The International journal of biochemistry. 14(2), 119–126 (1982)
Kessel, D., Allen, J.: Elevated plasma sialyltransferase in the cancer patient. Cancer research. 35(3), 670–672 (1975)
Bernacki, R.J., Kim, U.: Concomitant elevations in serum sialytransferase activity and sialic acid content in rats with metastasizing mammary tumors. Science. 195(4278), 577–580 (1977)
Dao, T.L., Ip, C., Patel, J.: Serum sialyltransferase and 5′-nucleotidase as reliable biomarkers in women with breast cancer. Journal of the National Cancer Institute. 65(3), 529–534 (1980)
Raval, G.N., Parekh, L.J., Patel, D.D., Jha, F.P., Sainger, R.N., Patel, P.S.: Clinical usefulness of alterations in sialic acid, sialyl transferase and sialoproteins in breast cancer. Indian journal of clinical biochemistry: IJCB. 19(2), 60–71 (2004). https://doi.org/10.1007/BF02894259
Raval, G.N., Patel, D.D., Parekh, L.J., Patel, J.B., Shah, M.H., Patel, P.S.: Evaluation of serum sialic acid, sialyltransferase and sialoproteins in oral cavity cancer. Oral diseases. 9(3), 119–128 (2003)
Miyagi, T., Takahashi, K., Hata, K., Shiozaki, K., Yamaguchi, K.: Sialidase significance for cancer progression. Glycoconjugate journal 29(8–9), 567–577 (2012). doi:https://doi.org/10.1007/s10719-012-9394-1
Hata, K., Koseki, K., Yamaguchi, K., Moriya, S., Suzuki, Y., Yingsakmongkon, S., Hirai, G., Sodeoka, M., von Itzstein, M., Miyagi, T.: Limited inhibitory effects of oseltamivir and zanamivir on human sialidases. Antimicrobial agents and chemotherapy 52(10), 3484–3491 (2008). doi:https://doi.org/10.1128/AAC.00344-08
Koseki, K., Wada, T., Hosono, M., Hata, K., Yamaguchi, K., Nitta, K., Miyagi, T.: Human cytosolic sialidase NEU2-low general tissue expression but involvement in PC-3 prostate cancer cell survival. Biochemical and biophysical research communications 428(1), 142–149 (2012). doi:https://doi.org/10.1016/j.bbrc.2012.10.028
Bilyy, R., Tomin, A., Mahorivska, I., Shalay, O., Lohinskyy, V., Stoika, R., Kit, Y.: Antibody-mediated sialidase activity in blood serum of patients with multiple myeloma. Journal of molecular recognition: JMR. 24(4), 576–584 (2011). https://doi.org/10.1002/jmr.1071
Kakugawa, Y., Wada, T., Yamaguchi, K., Yamanami, H., Ouchi, K., Sato, I., Miyagi, T.: Up-regulation of plasma membrane-associated ganglioside sialidase (Neu3) in human colon cancer and its involvement in apoptosis suppression. Proceedings of the National Academy of Sciences of the United States of America. 99(16), 10,718–10,723 (2002). https://doi.org/10.1073/pnas.152597199
Ueno, S., Saito, S., Wada, T., Yamaguchi, K., Satoh, M., Arai, Y., Miyagi, T.: Plasma membrane-associated sialidase is up-regulated in renal cell carcinoma and promotes interleukin-6-induced apoptosis suppression and cell motility. The Journal of biological chemistry. 281(12), 7756–7764 (2006). https://doi.org/10.1074/jbc.M509668200
Nomura, H., Tamada, Y., Miyagi, T., Suzuki, A., Taira, M., Suzuki, N., Susumu, N., Irimura, T., Aoki, D.: Expression of NEU3 (plasma membrane-associated sialidase) in clear cell adenocarcinoma of the ovary: its relationship with T factor of pTNM classification. Oncology research. 16(6), 289–297 (2006)
Kawamura, S., Sato, I., Wada, T., Yamaguchi, K., Li, Y., Li, D., Zhao, X., Ueno, S., Aoki, H., Tochigi, T., Kuwahara, M., Kitamura, T., Takahashi, K., Moriya, S., Miyagi, T.: Plasma membrane-associated sialidase (NEU3) regulates progression of prostate cancer to androgen-independent growth through modulation of androgen receptor signaling. Cell death and differentiation 19(1), 170–179 (2012). doi:https://doi.org/10.1038/cdd.2011.83
Hata, K., Tochigi, T., Sato, I., Kawamura, S., Shiozaki, K., Wada, T., Takahashi, K., Moriya, S., Yamaguchi, K., Hosono, M., Miyagi, T.: Increased sialidase activity in serum of cancer patients: Identification of sialidase and inhibitor activities in human serum. Cancer science 106(4), 383–389 (2015). doi:https://doi.org/10.1111/cas.12627
Sonmez, H., Suer, S., Gungor, Z., Baloglu, H., Kokoglu, E.: Tissue and serum sialidase levels in breast cancer. Cancer letters. 136(1), 75–78 (1999)
Klenk, E., Langerbeins, H.: Orcinol method for measuring sialic acid. Hoppe-Seyler’s Z Physiol Chem. 270, 185–193 (1941)
Svennerholm, L.: Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochimica et biophysica acta. 24(3), 604–611 (1957)
Aminoff, D.: Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. The Biochemical journal. 81, 384–392 (1961)
Warren, L.: The thiobarbituric acid assay of sialic acids. The Journal of biological chemistry. 234(8), 1971–1975 (1959)
Durand, G., Feger, J., Coignoux, M., Agneray, J., Pays, M.: Rapid estimation of small amounts of formaldehyde liberated during periodate oxidation of a sialoglycoprotein. Analytical biochemistry. 61(1), 232–236 (1974)
Massamiri, Y., Durand, G., Richard, A., Feger, J., Agneray, J.: Determination of erythrocyte surface sialic acid residues by a new colorimetric method. Analytical biochemistry. 97(2), 346–351 (1979)
Katopodis, N., Hirshaut, Y., Geller, N.L., Stock, C.C.: Lipid-associated sialic acid test for the detection of human cancer. Cancer research. 42(12), 5270–5275 (1982)
Hess, H., Rolde, E.: Fluorometric Assay of Sialic Acid in Brain Gangliosides. The Journal of biological chemistry. 239, 3215–3220 (1964)
Brunetti, P., Swanson, A., Roseman, S.: [68] Enzymatic determination of sialic acids: N-acylneuraminic acid N-acyl-d-mannosamine+ pyruvate. Methods in enzymology. 6, 465–473 (1963)
Rohrer, J.S.: Analyzing sialic acids using high-performance anion-exchange chromatography with pulsed amperometric detection. Analytical biochemistry 283(1), 3–9 (2000). doi:https://doi.org/10.1006/abio.2000.4643
Kamerling, J.P., Vliegenthart, J.F.: Gas-liquid chromatography and mass spectrometry of sialic acids. Springer, Sialic Acids (1982)
Schauer, R., Corfield, A.P.: Colorimetry and thin-layer chromatography of sialic acids. Springer, Sialic Acids (1982)
Shukla, A.K., Scholz, N., Reimerdes, E.H., Schauer, R.: High-performance liquid chromatography of N. O-acylated sialic acids. Analytical biochemistry. 123(1), 78–82 (1982)
Hara, S., Yamaguchi, M., Takemori, Y., Furuhata, K., Ogura, H., Nakamura, M.: Determination of mono-O-acetylated N-acetylneuraminic acids in human and rat sera by fluorometric high-performance liquid chromatography. Analytical biochemistry. 179(1), 162–166 (1989)
Klein, A., Diaz, S., Ferreira, I., Lamblin, G., Roussel, P., Manzi, A.E.: New sialic acids from biological sources identified by a comprehensive and sensitive approach: liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS) of SIA quinoxalinones. Glycobiology. 7(3), 421–432 (1997)
Shubhakar, A., Kozak, R.P., Reiding, K.R., Royle, L., Spencer, D.I., Fernandes, D.L., Wuhrer, M.: Automated High-Throughput Permethylation for Glycosylation Analysis of Biologics Using MALDI-TOF-MS. Analytical chemistry 88(17), 8562–8569 (2016). doi:https://doi.org/10.1021/acs.analchem.6b01639
Powell, A.K., Harvey, D.J.: Stabilization of sialic acids in N-linked oligosaccharides and gangliosides for analysis by positive ion matrix-assisted laser desorption/ionization mass spectrometry. Rapid communications in mass spectrometry: RCM 10(9), 1027–1032 (1996). doi:https://doi.org/10.1002/(SICI)1097-0231(19960715)10:9 < 1027::AID-RCM634 > 3.0.CO;2-Y.
Reiding, K.R., Blank, D., Kuijper, D.M., Deelder, A.M., Wuhrer, M.: High-throughput profiling of protein N-glycosylation by MALDI-TOF-MS employing linkage-specific sialic acid esterification. Analytical chemistry. 86(12), 5784–5793 (2014). https://doi.org/10.1021/ac500335t
Sekiya, S., Wada, Y., Tanaka, K.: Derivatization for stabilizing sialic acids in MALDI-MS. Analytical chemistry. 77(15), 4962–4968 (2005). https://doi.org/10.1021/ac050287o
Liu, X., Qiu, H., Lee, R.K., Chen, W., Li, J.: Methylamidation for sialoglycomics by MALDI-MS: a facile derivatization strategy for both alpha2,3- and alpha2,6-linked sialic acids. Analytical chemistry. 82(19), 8300–8306 (2010). https://doi.org/10.1021/ac101831t
de Haan, N., Reiding, K.R., Haberger, M., Reusch, D., Falck, D., Wuhrer, M.: Linkage-specific sialic acid derivatization for MALDI-TOF-MS profiling of IgG glycopeptides. Analytical chemistry. 87(16), 8284–8291 (2015). https://doi.org/10.1021/acs.analchem.5b02426
Wang, F., Xie, B., Wang, B., Troy, F.A., 2nd: LC-MS/MS glycomic analyses of free and conjugated forms of the sialic acids, Neu5Ac, Neu5Gc and KDN in human throat cancers. Glycobiology 25(12), 1362–1374 (2015). doi:https://doi.org/10.1093/glycob/cwv051
Inoue, S., Lin, S.L., Chang, T., Wu, S.H., Yao, C.W., Chu, T.Y., Troy 2nd, F.A., Inoue, Y.: Identification of free deaminated sialic acid (2-keto-3-deoxy-D-glycero-D-galacto-nononic acid) in human red blood cells and its elevated expression in fetal cord red blood cells and ovarian cancer cells. The Journal of biological chemistry. 273(42), 27,199–27,204 (1998)
Ikeda, K., Taguchi, R.: Highly sensitive localization analysis of gangliosides and sulfatides including structural isomers in mouse cerebellum sections by combination of laser microdissection and hydrophilic interaction liquid chromatography/electrospray ionization mass spectrometry with theoretically expanded multiple reaction monitoring. Rapid communications in mass spectrometry: RCM. 24(20), 2957–2965 (2010). https://doi.org/10.1002/rcm.4716
Shi, Y., Xu, X., Fang, M., Zhang, M., Li, Y., Gillespie, B., Yorke, S., Yang, N., McKew, J.C., Gahl, W.A., Huizing, M., Carrillo-Carrasco, N., Wang, A.Q.: Quantitative hydrophilic interaction chromatography-mass spectrometry analysis of N-acetylneuraminic acid and N-acetylmannosamine in human plasma. Journal of chromatography. B. Analytical technologies in the biomedical and life sciences. 1000, 105–111 (2015). https://doi.org/10.1016/j.jchromb.2015.07.018
Priego-Capote, F., Orozco-Solano, M.I., Calderon-Santiago, M.: Luque de Castro, M.D.: Quantitative determination and confirmatory analysis of N-acetylneuraminic and N-glycolylneuraminic acids in serum and urine by solid-phase extraction on-line coupled to liquid chromatography-tandem mass spectrometry. Journal of chromatography. A. 1346, 88–96 (2014). https://doi.org/10.1016/j.chroma.2014.04.051
Zeleny, R., Kolarich, D., Strasser, R., Altmann, F.: Sialic acid concentrations in plants are in the range of inadvertent contamination. Planta. 224(1), 222–227 (2006). https://doi.org/10.1007/s00425-005-0206-8
Singhal, A., Hakomori, S.: Molecular changes in carbohydrate antigens associated with cancer. BioEssays: news and reviews in molecular, cellular and developmental biology. 12(5), 223–230 (1990). https://doi.org/10.1002/bies.950120506
Wongkham, S., Bhudhisawasdi, V., Chau-in, S., Boonla, C., Muisuk, K., Kongkham, S., Wongkham, C., Boonsiri, P., Thuwajit, P.: Clinical significance of serum total sialic acid in cholangiocarcinoma. Clinica Chimica Acta; International Journal of Clinical Chemistry. 327(1–2), 139–147 (2003)
Ganzinger, U., Deutsch, E.: Serum sialyltransferase levels as a parameter in the diagnosis and follow-up of gastrointestinal tumors. Cancer research. 40(4), 1300–1304 (1980)
Griffiths, J., Reynolds, S.: Plasma sialyl transferase total and isoenzyme activity in the diagnosis of cancer of the colon. Clinical biochemistry. 15(1), 46–48 (1982)
Stefenelli, N., Klotz, H., Engel, A., Bauer, P.: Serum sialic acid in malignant tumors, bacterial infections, and chronic liver diseases. Journal of cancer research and clinical oncology. 109(1), 55–59 (1985)
Greville, G., McCann, A., Rudd, P.M., Saldova, R.: Epigenetic regulation of glycosylation and the impact on chemo-resistance in breast and ovarian cancer. Epigenetics 11(12), 845–857 (2016). doi:https://doi.org/10.1080/15592294.2016.1241932
Saldova, R., Dempsey, E., Perez-Garay, M., Marino, K., Watson, J.A., Blanco-Fernandez, A., Struwe, W.B., Harvey, D.J., Madden, S.F., Peracaula, R., McCann, A., Rudd, P.M.: 5-AZA-2′-deoxycytidine induced demethylation influences N-glycosylation of secreted glycoproteins in ovarian cancer. Epigenetics. 6(11), 1362–1372 (2011). https://doi.org/10.4161/epi.6.11.17977
Chachadi, V.B., Cheng, H., Klinkebiel, D., Christman, J.K., Cheng, P.W.: 5-Aza-2′-deoxycytidine increases sialyl Lewis X on MUC1 by stimulating beta-galactoside:alpha2,3-sialyltransferase 6 gene. The international journal of biochemistry & cell biology. 43(4), 586–593 (2011). https://doi.org/10.1016/j.biocel.2010.12.015
Crook, M.A., Treloar, A., Haq, M., Tutt, P.: Serum total sialic acid and acute phase proteins in elderly subjects. European journal of clinical chemistry and clinical biochemistry: journal of the Forum of European Clinical Chemistry Societies. 32(10), 745–747 (1994)
Taniuchi, K., Chifu, K., Hayashi, N., Nakamachi, Y., Yamaguchi, N., Miyamoto, Y., Doi, K., Baba, S., Uchida, Y., Tsukada, Y., Sugimori, T.: A new enzymatic method for the determination of sialic acid in serum and its application for a marker of acute phase reactants. The Kobe journal of medical sciences. 27(3), 91–102 (1981)
Blain, P.G., Mucklow, J.C., Rawlins, M.D., Roberts, D.F., Routledge, P.A., Shand, D.G.: Determinants of plasma alpha 1-acid glycoprotein (AAG) concentrations in health. British journal of clinical pharmacology. 20(5), 500–502 (1985)
Dage, J.L., Ackermann, B.L., Halsall, H.B.: Site localization of sialyl Lewis(x) antigen on alpha1-acid glycoprotein by high performance liquid chromatography-electrospray mass spectrometry. Glycobiology. 8(8), 755–760 (1998)
Wieruszeski, J.M., Fournet, B., Konan, D., Biou, D., Durand, G.: 400-MHz 1H-NMR spectroscopy of fucosylated tetrasialyl oligosaccharides isolated from normal and cirrhotic alpha 1-acid glycoprotein. FEBS letters. 238(2), 390–394 (1988)
Carter, A., Martin, N.H.: Serum sialic acid levels in health and disease. Journal of clinical pathology. 15, 69–72 (1962)
Horgan, I.E.: Total and lipid-bound sialic acid levels in sera from patients with cancer. Clinica Chimica Acta; International Journal of Clinical Chemistry. 118(2–3), 327–331 (1982)
Acknowledgements
We thank Alan Moran for carefully proof-reading this manuscript. The work was supported by the National Key Research and Development Program of China (No.2016YFA0501303; to Z.Z.) as well as the European Commission, Horizon 2020 Marie Skłodowska-Curie Actions “GlyCoCan” under grant agreement No. 676421 (to M.W. and S.H.) and “GlySign” under grant agreement No. 722095 (to M.W.). We also acknowledge the support received by Zejian Zhang from the China Scholarship Council (CSC) for PhD in the Netherlands/Europe (CSC Grant No. 201606100187).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zhang, Z., Wuhrer, M. & Holst, S. Serum sialylation changes in cancer. Glycoconj J 35, 139–160 (2018). https://doi.org/10.1007/s10719-018-9820-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-018-9820-0