Abstract
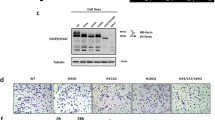
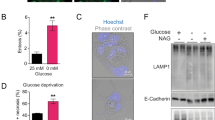
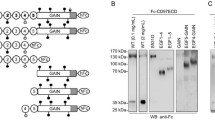
N-linked glycosylation (NLG) is a co-translational modification that is essential for the folding, stability, and trafficking of transmembrane (TM) and secretory glycoproteins. Efficient NLG requires the stepwise synthesis and en bloc transfer of a 14-sugar carbohydrate known as a lipid-linked oligosaccharide (LLO). The genetics of LLO biosynthesis have been established in yeast and Chinese hamster systems, but human models of LLO biosynthesis are lacking. In this study we report that Kato III human gastric cancer cells represent a model of deficient LLO synthesis, possessing a homozygous deletion of the LLO biosynthesis factor, MPDU1. Kato III cells lacking MPDU1 have all the hallmarks of a glycosylation-deficient cell line, including altered sensitivity to lectins and the formation of truncated LLOs. Analysis of transcription using an expression microarray and protein levels using a proteome antibody array reveal changes in the expression of several membrane proteins, including the metalloprotease ADAM-15 and the cell adhesion molecule CEACAM1. Surprisingly, the restoration of MPDU1 expression in Kato III cells demonstrated a clear phenotype of increased cell-cell adhesion, a finding that was confirmed in vivo through analysis of tumor xenografts. These experiments also confirmed that protein levels of CEACAM-1, which functions in cell adhesion, is dependent on LLO biosynthesis in vivo. Kato III cells and the MPDU1-rescued Kato IIIM cells therefore provide a novel model to examine the consequences of defective LLO biosynthesis both in vitro and in vivo.






Similar content being viewed by others
Abbreviations
- LLO :
-
Lipid-linked oligosaccharide
- NLG :
-
N-linked glycosylation
- ER :
-
Endoplasmic reticulum
- CHO :
-
Chinese hamster ovary
- CDG :
-
Congenital disorder of glycosylation
- MPDU :
-
Mannose-P-dolichol utilization
- Man :
-
Mannose
- GlcNac :
-
N-acetylglucosamine
- FACE :
-
Fluorophore-assisted carbohydrate electrophoresis
- CNV :
-
Copy number variation
- H&E :
-
Hematoxylin and eosin
- PBS :
-
Phosphate-buffered sailne
References
Anand, M., Rush, J.S., Ray, S., Doucey, M.A., Weik, J., Ware, F.E., Hofsteenge, J., Waechter, C.J., Lehrman, M.A.: Requirement of the Lec35 gene for all known classes of monosaccharide-P-dolichol-dependent glycosyltransferase reactions in mammals. Mol. Biol. Cell. 12, 487–501 (2001)
Baranov, V., Hammarstrom, S.: Carcinoembryonic antigen (CEA) and CEA-related cell adhesion molecule 1 (CEACAM1), apically expressed on human colonic M cells, are potential receptors for microbial adhesion. Histochem. Cell Biol. 121, 83–89 (2004)
Benchimol, S., Fuks, A., Jothy, S., Beauchemin, N., Shirota, K., Stanners, C.P.: Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell. 57, 327–334 (1989)
Cazet, A., Charest, J., Bennett, D.C., Sambrooks, C.L., Contessa, J.N.: Mannose phosphate isomerase regulates fibroblast growth factor receptor family signaling and glioma radiosensitivity. PLoS One. 9, e110345 (2014)
Chapman, A., Fujimoto, K., Kornfeld, S.: The primary glycosylation defect in class E Thy-1-negative mutant mouse lymphoma cells is an inability to synthesize dolichol-P-mannose. J. Biol. Chem. 255, 4441–4446 (1980)
Chorny, A., Cerutti, A.: CEACAM1-S: the virtues of alternative splicing in gut immunity. Immunity. 37, 768–770 (2012)
Cline, A., Gao, N., Flanagan-Steet, H., Sharma, V., Rosa, S., Sonon, R., Azadi, P., Sadler, K.C., Freeze, H.H., Lehrman, M.A., et al.: A zebrafish model of PMM2-CDG reveals altered neurogenesis and a substrate-accumulation mechanism for N-linked glycosylation deficiency. Mol. Biol. Cell. 23, 4175–4187 (2012)
Contessa, J.N., Bhojani, M.S., Freeze, H.H., Rehemtulla, A., Lawrence, T.S.: Inhibition of N-linked glycosylation disrupts receptor tyrosine kinase signaling in tumor cells. Cancer Res. 68, 3803–3809 (2008)
Contessa, J.N., Bhojani, M.S., Freeze, H.H., Ross, B.D., Rehemtulla, A., Lawrence, T.S.: Molecular imaging of N-linked glycosylation suggests glycan biosynthesis is a novel target for cancer therapy. Clin. Cancer Res. Off J Am Assoc Cancer Res. 16, 3205–3214 (2010)
Gao, N., Lehrman, M.A.: Analyses of dolichol pyrophosphate-linked oligosaccharides in cell cultures and tissues by fluorophore-assisted carbohydrate electrophoresis. Glycobiology. 12, 353–360 (2002)
Haga, Y., Ishii, K., Suzuki, T.: N-glycosylation is critical for the stability and intracellular trafficking of glucose transporter GLUT4. J. Biol. Chem. 286, 31320–31327 (2011)
Hori, H., Elbein, A.D.: Characterization of the oligosaccharides from lipid-linked oligosaccharides of mung bean seedlings. Plant Physiol. 70, 12–20 (1982)
Huang, P., Chen, C., Mague, S.D., Blendy, J.A., Liu-Chen, L.Y.: A common single nucleotide polymorphism A118G of the mu opioid receptor alters its N-glycosylation and protein stability. Biochem. J. 441, 379–386 (2012)
Jones, M.B., Tomiya, N., Betenbaugh, M.J., Krag, S.S.: Analysis and metabolic engineering of lipid-linked oligosaccharides in glycosylation-deficient CHO cells. Biochem. Biophys. Res. Commun. 395, 36–41 (2010)
Karam, R., Carvalho, J., Bruno, I., Graziadio, C., Senz, J., Huntsman, D., Carneiro, F., Seruca, R., Wilkinson, M.F., Oliveira, C.: The NMD mRNA surveillance pathway downregulates aberrant E-cadherin transcripts in gastric cancer cells and in CDH1 mutation carriers. Oncogene. 27, 4255–4260 (2008)
Kranz, C., Denecke, J., Lehrman, M.A., Ray, S., Kienz, P., Kreissel, G., Sagi, D., Peter-Katalinic, J., Freeze, H.H., Schmid, T., Jackowski-Dohrmann, S., Harms, E., Marquardt, T.: A mutation in the human MPDU1 gene causes congenital disorder of glycosylation type If (CDG-If). J. Clin. Invest. 108, 1613–1619 (2001)
Lehrman, M.A., Zeng, Y.: Pleiotropic resistance to glycoprotein processing inhibitors in Chinese hamster ovary cells. The role of a novel mutation in the asparagine-linked glycosylation pathway. J. Biol. Chem. 264, 1584–1593 (1989)
Liem, Y.S., Bode, L., Freeze, H.H., Leebeek, F.W., Zandbergen, A.A., Paul, W.J.: Using heparin therapy to reverse protein-losing enteropathy in a patient with CDG-Ib. Nat. Clin. Pract. Gastroenterol. Hepatol. 5, 220–224 (2008)
Lucka, L., Fernando, M., Grunow, D., Kannicht, C., Horst, A.K., Nollau, P., Wagener, C.: Identification of Lewis x structures of the cell adhesion molecule CEACAM1 from human granulocytes. Glycobiology. 15, 87–100 (2005)
Maeda, Y., Tomita, S., Watanabe, R., Ohishi, K., Kinoshita, T.: DPM2 regulates biosynthesis of dolichol phosphate-mannose in mammalian cells: correct subcellular localization and stabilization of DPM1, and binding of dolichol phosphate. EMBO J. 17, 4920–4929 (1998)
Obrink, B.: CEA adhesion molecules: multifunctional proteins with signal-regulatory properties. Curr. Opin. Cell Biol. 9, 616–626 (1997)
Patnaik, S.K., Stanley, P.: Lectin-resistant CHO glycosylation mutants. Methods Enzymol. 416, 159–182 (2006)
Rush, J.S., Panneerselvam, K., Waechter, C.J., Freeze, H.H.: Mannose supplementation corrects GDP-mannose deficiency in cultured fibroblasts from some patients with Congenital Disorders of Glycosylation (CDG). Glycobiology. 10, 829–835 (2000)
Schenk, B., Imbach, T., Frank, C.G., Grubenmann, C.E., Raymond, G.V., Hurvitz, H., Korn-Lubetzki, I., Revel-Vik, S., Raas-Rotschild, A., Luder, A.S., et al.: MPDU1 mutations underlie a novel human congenital disorder of glycosylation, designated type If. J. Clin. Invest. 108, 1687–1695 (2001)
Sekiguchi, M., Sakakibara, K., Fujii, G.: Establishment of cultured cell lines derived from a human gastric carcinoma. Jpn J Exp Med. 48, 61–68 (1978)
Stoll, J., Rosenwald, A.G., Krag, S.S.: A Chinese hamster ovary cell mutant F2A8 utilizes polyprenol rather than dolichol for its lipid-dependent asparagine-linked glycosylation reactions. J. Biol. Chem. 263, 10774–10782 (1988)
Sun, L., Eklund, E.A., Van Hove, J.L., Freeze, H.H., Thomas, J.A.: Clinical and molecular characterization of the first adult congenital disorder of glycosylation (CDG) type Ic patient. Am. J. Med. Genet. A. 137, 22–26 (2005)
Ware, F.E., Lehrman, M.A.: Expression cloning of a novel suppressor of the Lec15 and Lec35 glycosylation mutations of Chinese hamster ovary cells. J. Biol. Chem. 271, 13935–13938 (1996)
Funding
This work was supported by grants from the National Institutes of Health (5RO1CA172391) and the National Research Service Award (postdoctoral training grant #T32CA009259).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Bennett, D.C., Cazet, A., Charest, J. et al. MPDU1 regulates CEACAM1 and cell adhesion in vitro and in vivo. Glycoconj J 35, 265–274 (2018). https://doi.org/10.1007/s10719-018-9819-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-018-9819-6