Abstract
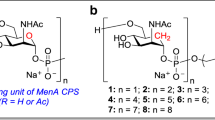
Multicomponent constructs, obtained by coupling different glycans to the carrier protein, have been proposed as a way to co-deliver multiple surface carbohydrates targeting different strains of one pathogen and reduce the number of biomolecules in the formulation of multivalent vaccines. To assess the feasibility of this approach for anti-microbial vaccines and investigate the potential immunodominance of one carbohydrate antigen over the others in these constructs, we designed a bivalent unimolecular vaccine against serogroup A (MenA) and C (MenC) meningococci, with the two different oligomers conjugated to same molecule of carrier protein (CRM197). The immune response elicited in mice by the bivalent MenAC construct was compared with the ones induced by the monovalent MenA and MenC vaccines and their combinations. After the second dose, the bivalent construct induced good levels of anti-MenA and anti-MenC antibodies with respect to the controls. However, the murine sera from the MenAC construct exhibited good anti-MenC bactericidal activity, and very low anti-MenA functionality when compared to the monovalent controls. This result was explained with the diverse relative avidities against MenA and MenC polysaccharides, which were measured in the generated sera. The immunodominant effect of the MenC antigen was fully overcome following the third immunization, when sera endowed with higher avidity and excellent bactericidal activity against both MenA and MenC expressing strains were elicited. Construction of multicomponent glycoconjugate vaccines against microbial pathogens is a feasible approach, but particular attention should be devoted to study and overcome possible occurrence of immune interference among the carbohydrates.





Similar content being viewed by others
References
Avci, F.Y., Kasper, D.L.: How bacterial carbohydrates influence the adaptive immune system. Annu Rev Immunol 28, 107–110 (2010)
Pace, D.: Glycoconjugate vaccines. Expert Opin Biol Ther 13, 11–33 (2013)
Calix. J.J. Porambo, R.J. Brady A.M. Larson. T.R. Yother J. Abeygunwardana C. Nahm M.H. Biochemical, genetic, and serological characterization of two capsule subtypes among Streptococcus pneumoniae serotype 20 strains: discovery of a new pneumococcal serotype. J. Biol. Chem., 27885–27894 (2012)
Delrieu, I., Yaro, S., Tamekloe, T.A., Njanpop-Lafourcade, B.M., Tall, H., Jaillard, P., Quedraogo, M.S., Badziclou, K., Sanou, O., Drabo, A., Gessner, B.D., Kambou, J.L., Mueller, J.E.: Neisseria meningitidis serogroup X meningitis in Togo and Burkina Faso. PLoS One 6, e19513 (2011)
Adamo, R., Nilo, A., Castagner, B., Boutureira, O., Berti, F., Bernardes, G.J.L.: Synthetically defined glycoprotein vaccines: current status & future directions. Chem Sci 4, 2995–3008 (2013)
Bardotti, A., Averani, G., Berti, F., Berti, S., Carinci, V., D’Ascenzi, S., Fabbri, B., Giannini, S., Giannozzi, A., Magagnoli, C., Proietti, D., Norelli, F., Rappuoli, R., Ricci, S., Costantino, P.: Physicochemical characterisation of glycoconjugate vaccines for prevention of meningococcal diseases. Vaccine 26, 2284–2296 (2009)
Porro, M., Costantino, P., Giovannoni, F., Pellegrini, V., Tagliaferri, L., Vannozzi, F., Viti, S.: A molecular model of artificial glycoprotein with predetermined multiple immunodeterminants for gram-positive and gram-negative encapsulated bacteria. Mol Immunol 23, 385–391 (1986)
Bongat, A.F.G., Saksena, R., Adamo, R., Fujimoto, Y., Shiokawa, Z., Peterson, D.C., Fukase, K., Vann, W.F., Kováč, P.: Multimeric bivalent immunogens from recombinant tetanus toxin HC fragment, synthetic hexasaccharides, and a glycopeptide adjuvant. Glycoconj J 27, 69–77 (2010)
Lipinski, T., Fitieh, A., St Pierre, J., Ostergaard, H.L., Bundle, D.R., Toure, N.: Enhanced immunogenicity of a tricomponent mannan tetanus Toxoid conjugate vaccine targeted to dendritic cells via dectin-1 by incorporating β glucan. J Infect Dis 190, 4116–4128 (2013)
Buskas, T., Thompson, P., Boons, G.-J.: Immunotherapy for cancer: synthetic carbohydrate-based vaccines. Chem. Comm., 5335–5349 (2009).
Ingale, S., Wolfert, M.A., Gaekwad, J., Buskas, T., Boons, G.-J.: Robust immune responses elicited by a fully synthetic three-component vaccine. Nat Chem Biol 3, 663–667 (2007)
Slovin, S.F., Ragupathi, G., Musselli, C., Fernandez, C., Diani, M., Verbel, D., Danishefsky, S., Livingston, P., Scher, H.: Thomsen-Friedenreich (TF) antigen as a target for prostate cancer vaccine: clinical trial results with TF cluster (c)-KLH plus QS21 conjugate vaccine in patients with biochemically relapsed prostate cancer. Cancer Immunol Immunother 54, 694–702 (2005)
Sant, A.J., Chaves, F.A., Krafcic, F.R., Lazarski, C.A., Menges, P., Richards, K., Weaver, J.M.: Immunodominance in CD4 T-cells response: implcations for immune responses to influenza virus for vaccine design. Expert Rev Vaccines 6, 367–368 (2007)
Chen, W., McCluskey, J. Immunodominance and Immunodomination: Critical Factors in Developing Effective CD8 T-Cell–Based Cancer Vaccines. Advances in Cancer Research, 203–248 (2006).
Gotschlich, E.C.: Meningococcal meningitis. In: Bacteria/ Vaccine. Ed. Germanier, R. Academic Press, New York, 237–255 (1984).
Lapeyssonnie, L.: Bull. World. Health Organ 28, 114 (1963)
Roberts, L.: Vaccine introduction. the beginning of the end for Africa's devastating meningitis outbreaks? Science 330, 1466–1467 (2010)
Clements, D.A., Gilbert, G.L.: Increase in admissions for Neisseria meningitidis infection in Australia. Lancet II, 1464 (1989).
Cohn, A.C., MacNeil, J.R., Harrison, L.H., Hatcher, C., Theodore, J., Schmidt, M., Pondo, T., Arnold, K.E., Baumbach, J., Bennett, N., Craig, A.S., Farley, M., Gershman, K., Petit, S., Lynfield, R., Reingold, A., Schaffner, W., Shutt, K.A., Zell, E.R., Mayer, L.W., Clark, T., Stephens, D., Messonnier, N.E.: Changes in Neisseria meningitidis disease epidemiology in the United States, 1998–2007: implications for prevention of meningococcal disease. Clin Infect Dis 50, 184–191 (2010)
Barroso, D.E., Castiñeiras, T.M.P.P., Freitas, F.S., Marsh, J.W., Krauland, M.G., Tulenko, M.M., Fonseca, É.L., Vicente, A.C.P., Rebelo, M.C., Cerqueira, E.O., Xavier, A.C., Cardozo, A.P.C.M., da Silva, S.E.M., Harrison, L.H.: Three outbreakcausing Neisseria meningitidis serogroup C clones,Brazil. Emerg Infect Dis 19, 1847–1850 (2013)
not listed], N.a.: Menactra: a meningococcal conjugate vaccine. Med. Lett. Drugs Ther. 47, 29–31 (2005).
not listed], N.a.: A new conjugate meningococcal vaccine (Menveo). Med. Lett. Drugs Ther. 52, 59–60 (2010).
Sow, S.O., Okoko, B.J., Diallo, A., Viviani, S., Borrow, R., Carlone, G., Tapia, M., Akinsola, A.K., Arduin, P., Findlow, H., Elie, C., Haidara, F.C., Adegbola, R.A., Diop, D., Parulekar, V., Chaumont, J., Martellet, L., Diallo, F., Idoko, O.T., Tang, Y., Plikaytis, B.D., Kulkarni, P.S., Marchetti, E., La Force, F.M., Preziosi, M.P.: Immunogenicity and safety of a meningococcal a conjugate vaccine in Africans. N Engl J Med 364, 2293–2304 (2011)
Costantino, P., Viti, S., Podda, A.M., Velmonte, A., Nencioni, L., Rappuoli, R.: Development and phase I clinical testing of a conjugate vaccine against meningococcus A and C. Vaccine 10, 691–698 (1992)
Costantino, P., Norelli, F., Giannozzi, A., D’Ascenzi, S., Bartoloni, A., Kaurb, S., Tang, D., Seid, R., Viti, S., Paffetti, R., Bigio, M., Pennatini, C., Averani, G., Guarnieri, V., Gallo, E., Ravenscroft, N., Lazzeroni, C., Rappuoli, R., Ceccarini, C.: Size fractionation of bacterial capsular polysaccharides for their use in conjugate vaccines. Vaccine 17, 1251–1263 (1999)
Macdonald, R.A., Hosking, C.S., Jones, C.L.: The measurement of relative antibody affinity by ELISA using thiocyanate elution. J Immunol Meth 106, 191–194 (1988)
Pullen, G.R., Fitzgerald, M.G., Hosking, C.S.: Antibody avidity determination by ELISA using thiocyanate elution. J Immunol Meth 86, 83–87 (1986)
Giuliani, M.M., Santini, L., Brunelli, B., Biolchi, A., Aricò, B., Di Marcello, F., Cartocci, E., Comanducci, M., Masignani, V., Lozzi, L., Savino, S., Scarselli, M., Rappuoli, R., Pizza, M.: The region comprising amino acids 100 to 255 of Neisseria meningitidis lipoprotein GNA 1870 elicits bactericidal antibodies. Infect Imm 73, 1151–1160 (2005)
Malito, E., Bursulaya, B., Chen, C., Lo Surdo, P., Picchianti, M., Balducci, E., Biancucci, M., Brock, A., Berti, F., Bottomley, M.J., Nissum, M., Costantino, P., Rappuoli, R., Spraggon, G.: Structural basis for lack of toxicity of the diphtheria toxin mutant. CRM197. Proc Natl Acad Sci U S A 190, 5229–5234 (2012)
Crotti, S., Zhai, H., Zhou, J., Allan, M., Proietti, D., Pansegrau, W., Hu, Q.-Y., Berti, F., Adamo, R.: Defined conjugation of glycans to the lysines of CRM197 guided by their reactivity mapping. Chembiochem Europ J chem biol 15, 836–843 (2014)
Vecchi, S., Bufali, S., Skibinski, D.A.G., O’Hagan, D.T., Singh, M.: Aluminum adjuvant dose guidelines in vaccine formulation for preclinical evaluations. J Pharm Sc 101, 17–20 (2012)
Campbell, H., Andrews, N., Borrow, R., Trotter, C., Miller, E.: Updated postlicensure surveillance of the meningococcal C conjugate vaccine in England and Wales: effectiveness, validation of serological correlates of protection, and modeling predictions of the duration of herd immunity. Clin Vaccine Immunol 17, 840–847 (2010)
Hirve, S., Bavdekar, A., Pandit, A., Juvekar, S., Patil, M., Preziosi, M.-P., Tang, Y., Marchetti, E., Martellet, L., Findlow, H., Elie, C., Parulekar, V., Plikaytis, B., Borrow, R., Carlone, G., Kulkarni, P.S., Goel, A., Suresh, K., Beri, S., Kapre, S., Jadhav, S., Preaud, J.-M., Viviani, S., LaForce, F.M.: Immunogenicity and safety of a new meningococcal a conjugate vaccine in Indian children aged 2–10 years: A Phase II/III double-blind randomized controlled trial. Vaccine 30, 6456–6460 (2012)
Micoli, F., Romano, M.R., Tontini, M., Cappelletti, E., Gavinia, M., Proietti, D., Rondini, S., Swennen, E., Santini, L., Filippini, S., Balocchi, C., Adamo, R., Pluschke, G., Norheimd, G., Pollard, A., Saul, A., Rappuoli, R., MacLennan, C.A., Berti, F., Costantino, P.: Development of a glycoconjugate vaccine to prevent meningitis in Africa caused by meningococcal serogroup X. Proc Natl Acad Sci U S A 110, 19077–19082 (2013)
Anttila, M., Voutilainen, M., Jaèntti, V., Eskola, J., Kaèythy, H.: Contribution of serotype-speci®c IgG concentration IgG subclasses and relative antibody avidity to opsonophagocytic activity against Streptococcus pneumoniae. Clin Exp Immunol 118, 402–407 (1999)
McKee, A.S., Munks, M.W., MacLeod, M.K.L., Fleenor, C.J., Van Rooijen, N., Kappler, J.W., Marrack, P.: Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol 183, 4403–4414 (2009)
Tontini, M., Berti, F., Romano, M.R., Proietti, D., Zambonelli, C., Bottomley, M.J., De Gregorio, E., Del Giudice, G., Rappuoli, R., Costantino, P.: Comparison of CRM197, diphtheria toxoid and tetanus toxoid as protein carriers for meningococcal glycoconjugate vaccines. Vaccine 31, 4827–4833 (2013)
Gilewski, T.A., Ragupathi, G., Dickler, M., Powell, S., Bhuta, S., Panageas, K.R., Koganty, R., Chin-Eng, J., Hudis, C., Norton, L., Houghton, A.N., Livingston, P.O.: Clin Cancer Res 13, 2977–2985 (2007)
Dagan, R., Poolman, J., Siegrist, C.A.: Glycoconjugate vaccines and immune interference: a review. Vaccine 28, 5513–5523 (2010)
Pollabauer, E.M., Petermann, R., Ehrlich, H.J.: The influence of carrier protein on the immunogenicity of simultaneously administered conjugate vaccines in infants. Vaccine 27, 1674–1679 (2009)
Barington, T., Skettrup, M., Juul, L., Heilmann, C.: Non-epitope specific suppression of the antibody response to Haemophilus influenzae type b conjugate vaccine by preimmunization with vaccine components. Infect Imm 61, 432–438 (1991)
Granoff, D.M., Holmes, S.J., Belshe, R.B., Osterholm, M.T., McHugh, J.E., Anderson, E.L.: Effect of carrier protein priming on antibody responses to Haemophilus influenzae type b conjugate vaccines in infants. J Am Med Assoc 272, 1116–1121 (1994)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adamo, R., Nilo, A., Harfouche, C. et al. Investigating the immunodominance of carbohydrate antigens in a bivalent unimolecular glycoconjugate vaccine against serogroup A and C meningococcal disease. Glycoconj J 31, 637–647 (2014). https://doi.org/10.1007/s10719-014-9559-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-014-9559-1