Abstract
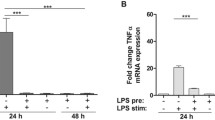
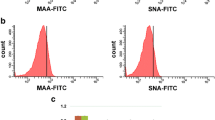
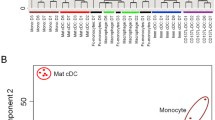
Using a focused glycan-gene microarray, we compared the glycosyltransferase (GT) and sulfotransferase gene expression profiles of human monocytes, dendritic cells (DCs) and macrophages (Mϕs), isolated or differentiated from the same donors. Microarray analysis indicated that monocytes express transcripts for a full set of enzymes involved in the biosynthesis of multi-multiantennary branched N-glycans, potentially elongated by poly-N-acetyl-lactosamine chains, and of mucin-type Core 1 and Core 2 sialylated O-glycans. Monocytes also express genes involved in the biosynthesis and modification of glycosaminoglycans, but display a limited expression of GTs implicated in glycolipid synthesis. Among genes expressed in monocytes (90 out of 175), one third is significantly modulated in DCs and Mϕ respectively, most of them being increased in both cell types relative to monocytes. These changes might potentially enforce the capacity of differentiated cells to synthesize branched N-glycans and mucin-type O-glycans and to remodel cell surface proteoglycans. Stimulation of DCs and Mϕs with lipopolysaccharide caused a general decrease in gene expression, mainly affecting genes found to be positively modulated during the differentiation steps. Interestingly, although a similar set of enzymes are modulated in the same direction in mature DCs and Mϕs, cell specific genes are also differentially regulated during maturation, a phenomenon that may sustain functional specificities. Validation of this analysis was provided by quantitative real-time PCR and flow cytometry of cell surface glycan antigens. Collectively, this study implies an important modification of the pattern of glycosylation in DCs and Mϕs undergoing differentiation and maturation with potential biological consequences.


Similar content being viewed by others
Abbreviations
- APC:
-
Antigen presenting cell
- DC:
-
Dendritic cell
- Ag:
-
Antigen
- GSL:
-
Glycosphingolipid
- GT:
-
Glycosyltransferase
- Mϕ:
-
Macrophage
- TLR:
-
Toll-like receptor
- sLex :
-
Sialyl Lex
- GAG:
-
Glycosaminoglycan
- ST:
-
Sialyltransferase
- PSA:
-
Polysialic acid
- HS:
-
Heparan sulfate
- qPCR:
-
Quantitative real-time PCR
- LPS:
-
Lipopolysaccharide
- MFI:
-
Mean fluorescence intensity
- GM-CSF:
-
Granulocyte Mϕ-colony stimulating factor
References
Arnold, J.N., Wormald, M.R., Sim, R.B., Rudd, P.M., Dwek, R.A.: The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol. 25, 21–50 (2007). doi:10.1146/annurev.immunol.25.022106.141702
Collins, B.E., Paulson, J.C.: Cell surface biology mediated by low affinity multivalent protein-glycan interactions. Curr. Opin. Chem. Biol. 8, 617–625 (2004). doi:10.1016/j.cbpa.2004.10.004
Daniels, M.A., Hogquist, K.A., Jameson, S.C.: Sweet ’n’ sour: the impact of differential glycosylation on T cell responses. Nat. Immunol. 3, 903–910 (2002). doi:10.1038/ni1002-903
Marth, J.D., Grewal, P.K.: Mammalian glycosylation in immunity. Nat. Rev. Immunol. 8, 874–887 (2008). doi:10.1038/nri2417
Rudd, P.M., Elliott, T., Cresswell, P., Wilson, I.A., Dwek, R.A.: Glycosylation and the immune system. Science 291, 2370–2376 (2001). doi:10.1126/science.291.5512.2370
Spiro, R.G.: Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology 12, 43R–56R (2002). doi:10.1093/glycob/12.4.43R
Crocker, P.R.: Siglecs: sialic-acid-binding immunoglobulin-like lectins in cell-cell interactions and signalling. Curr. Opin. Struct. Biol. 12, 609–615 (2002). doi:10.1016/S0959-440X(02)00375-5
Esko, J.D., Selleck, S.B.: Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu. Rev. Biochem. 71, 435–471 (2002). doi:10.1146/annurev.biochem.71.110601.135458
Lau, K.S., Partridge, E.A., Grigorian, A., Silvescu, C.I., Reinhold, V.N., Demetriou, M., Dennis, J.W.: Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell 129, 123–134 (2007). doi:10.1016/j.cell.2007.01.049
Lowe, J.B.: Glycosyltransferases and glycan structures contributing to the adhesive activities of L-, E- and P-selectin counter-receptors. Biochem. Soc. Symp. 69, 33–45 (2002)
Moody, A.M., Chui, D., Reche, P.A., Priatel, J.J., Marth, J.D., Reinherz, E.L.: Developmentally regulated glycosylation of the CD8alphabeta coreceptor stalk modulates ligand binding. Cell 107, 501–512 (2001). doi:10.1016/S0092-8674(01)00577-3
Rabinovich, G.A., Baum, L.G., Tinari, N., Paganelli, R., Natoli, C., Liu, F.T., Iacobelli, S.: Galectins and their ligands: amplifiers, silencers or tuners of the inflammatory response? Trends Immunol. 23, 313–320 (2002). doi:10.1016/S1471-4906(02)02232-9
Toscano, M.A., Bianco, G.A., Ilarregui, J.M., Croci, D.O., Correale, J., Hernandez, J.D., Zwirner, N.W., Poirier, F., Riley, E.M., Baum, L.G., Rabinovich, G.A.: Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat. Immunol. 8, 825–834 (2007). doi:10.1038/ni1482
Blander, J.M., Visintin, I., Janeway Jr., C.A., Medzhitov, R.: Alpha(1, 3)-fucosyltransferase VII and alpha(2, 3)-sialyltransferase IV are up-regulated in activated CD4 T cells and maintained after their differentiation into Th1 and migration into inflammatory sites. J. Immunol. 163, 3746–3752 (1999)
Collins, B.E., Blixt, O., Han, S., Duong, B., Li, H., Nathan, J.K., Bovin, N., Paulson, J.C.: High-affinity ligand probes of CD22 overcome the threshold set by cis ligands to allow for binding, endocytosis, and killing of B cells. J. Immunol. 177, 2994–3003 (2006)
Feizi, T.: Carbohydrate-mediated recognition systems in innate immunity. Immunol. Rev. 173, 79–88 (2000). doi:10.1034/j.1600-065X.2000.917310.x
Moody, A.M., North, S.J., Reinhold, B., Van Dyken, S.J., Rogers, M.E., Panico, M., Dell, A., Morris, H.R., Marth, J.D., Reinherz, E.L.: Sialic acid capping of CD8beta core 1-O-glycans controls thymocyte-major histocompatibility complex class I interaction. J. Biol. Chem. 278, 7240–7246 (2003). doi:10.1074/jbc.M210468200
Morgan, R., Gao, G., Pawling, J., Dennis, J.W., Demetriou, M., Li, B.: N-acetylglucosaminyltransferase V (Mgat5)-mediated N-glycosylation negatively regulates Th1 cytokine production by T cells. J. Immunol. 173, 7200–7208 (2004)
Pappu, B.P., Shrikant, P.A.: Alteration of cell surface sialylation regulates antigen-induced naive CD8+ T cell responses. J. Immunol. 173, 275–284 (2004)
van Kooyk, Y., Rabinovich, G.A.: Protein-glycan interactions in the control of innate and adaptive immune responses. Nat. Immunol. 9, 593–601 (2008). doi:10.1038/ni.f.203
Demetriou, M., Granovsky, M., Quaggin, S., Dennis, J.W.: Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature 409, 733–739 (2001). doi:10.1038/35055582
Moretta, L., Bottino, C., Pende, D., Castriconi, R., Mingari, M.C., Moretta, A.: Surface NK receptors and their ligands on tumor cells. Semin. Immunol. 18, 151–158 (2006). doi:10.1016/j.smim.2006.03.002
Bendelac, A., Savage, P.B., Teyton, L.: The biology of NKT cells. Annu. Rev. Immunol. 25, 297–336 (2007). doi:10.1146/annurev.immunol.25.022106.141711
Godfrey, D.I., Kronenberg, M.: Going both ways: immune regulation via CD1d-dependent NKT cells. J. Clin. Invest. 114, 1379–1388 (2004)
Bax, M., Garcia-Vallejo, J.J., Jang-Lee, J., North, S.J., Gilmartin, T.J., Hernandez, G., Crocker, P.R., Leffler, H., Head, S.R., Haslam, S.M., Dell, A., van Kooyk, Y.: Dendritic cell maturation results in pronounced changes in glycan expression affecting recognition by siglecs and galectins. J. Immunol. 179, 8216–8224 (2007)
Julien, S., Grimshaw, M.J., Sutton-Smith, M., Coleman, J., Morris, H.R., Dell, A., Taylor-Papadimitriou, J., Burchell, J.M.: Sialyl-Lewis(x) on P-selectin glycoprotein ligand-1 is regulated during differentiation and maturation of dendritic cells: a mechanism involving the glycosyltransferases C2GnT1 and ST3Gal I. J. Immunol. 179, 5701–5710 (2007)
Gordon, S., Taylor, P.R.: Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 (2005). doi:10.1038/nri1733
Hume, D.A., Ross, I.L., Himes, S.R., Sasmono, R.T., Wells, C.A., Ravasi, T.: The mononuclear phagocyte system revisited. J. Leukoc. Biol. 72, 621–627 (2002)
Randolph, G.J., Beaulieu, S., Lebecque, S., Steinman, R.M., Muller, W.A.: Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science 282, 480–483 (1998). doi:10.1126/science.282.5388.480
Randolph, G.J., Inaba, K., Robbiani, D.F., Steinman, R.M., Muller, W.A.: Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity 11, 753–761 (1999). doi:10.1016/S1074-7613(00)80149-1
Banchereau, J., Steinman, R.M.: Dendritic cells and the control of immunity. Nature 392, 245–252 (1998). doi:10.1038/32588
Kapsenberg, M.L.: Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 3, 984–993 (2003). doi:10.1038/nri1246
Reis e Sousa, C.: Dendritic cells in a mature age. Nat. Rev. Immunol. 6, 476–483 (2006). doi:10.1038/nri1845
Rossi, M., Young, J.W.: Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J. Immunol. 175, 1373–1381 (2005)
Iwasaki, A., Medzhitov, R.: Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5, 987–995 (2004). doi:10.1038/ni1112
Takeda, K., Akira, S.: Toll-like receptors in innate immunity. Int. Immunol. 17, 1–14 (2005). doi:10.1093/intimm/dxh186
Gosset, P., Bureau, F., Angeli, V., Pichavant, M., Faveeuw, C., Tonnel, A.B., Trottein, F.: Prostaglandin D2 affects the maturation of human monocyte-derived dendritic cells: consequence on the polarization of naive Th cells. J. Immunol. 170, 4943–4952 (2003)
Sallusto, F., Lanzavecchia, A.: Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179, 1109–1118 (1994). doi:10.1084/jem.179.4.1109
Young, D.A., Lowe, L.D., Clark, S.C.: Comparison of the effects of IL-3, granulocyte-macrophage colony-stimulating factor, and macrophage colony-stimulating factor in supporting monocyte differentiation in culture. Analysis of macrophage antibody-dependent cellular cytotoxicity. J. Immunol. 145, 607–615 (1990)
Turville, S.G., Cameron, P.U., Handley, A., Lin, G., Pohlmann, S., Doms, R.W., Cunningham, A.L.: Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 3, 975–983 (2002). doi:10.1038/ni841
van Kooyk, Y., Geijtenbeek, T.B.: DC-SIGN: escape mechanism for pathogens. Nat. Rev. Immunol. 3, 697–709 (2003). doi:10.1038/nri1182
Fleetwood, A.J., Lawrence, T., Hamilton, J.A., Cook, A.D.: Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J. Immunol. 178, 5245–5252 (2007)
Verreck, F.A., de Boer, T., Langenberg, D.M., Hoeve, M.A., Kramer, M., Vaisberg, E., Kastelein, R., Kolk, A., de Waal-Malefyt, R., Ottenhoff, T.H.: Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco) bacteria. Proc. Natl. Acad. Sci. USA 101, 4560–4565 (2004). doi:10.1073/pnas.0400983101
Lockhart, D.J., Dong, H., Byrne, M.C., Follettie, M.T., Gallo, M.V., Chee, M.S., Mittmann, M., Wang, C., Kobayashi, M., Horton, H., Brown, E.L.: Expression monitoring by hybridization to high-density oligonucleotide arrays. Nat. Biotechnol. 14, 1675–1680 (1996). doi:10.1038/nbt1296-1675
Hess, A., Iyer, H.: Fisher’s combined p-value for detecting differentially expressed genes using Affymetrix expression arrays. BMC Genomics 8, 96 (2007). doi:10.1186/1471-2164-8-96
Bolstad, B.M., Irizarry, R.A., Astrand, M., Speed, T.P.: A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19, 185–193 (2003). doi:10.1093/bioinformatics/19.2.185
Irizarry, R.A., Bolstad, B.M., Collin, F., Cope, L.M., Hobbs, B., Speed, T.P.: Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 31, e15 (2003). doi:10.1093/nar/gng015
Elbim, C., Hakim, J., Gougerot-Pocidalo, M.A.: Heterogeneity in Lewis-X and sialyl-Lewis-X antigen expression on monocytes in whole blood: relation to stimulus-induced oxidative burst. Am. J. Pathol. 152, 1081–1090 (1998)
Skacel, P.O., Edwards, A.J., Harrison, C.T., Watkins, W.M.: Enzymic control of the expression of the X determinant (CD15) in human myeloid cells during maturation: the regulatory role of 6-sialytransferase. Blood 78, 1452–1460 (1991)
Becker, S., Warren, M.K., Haskill, S.: Colony-stimulating factor-induced monocyte survival and differentiation into macrophages in serum-free cultures. J. Immunol. 139, 3703–3709 (1987)
Inaba, K., Inaba, M., Romani, N., Aya, H., Deguchi, M., Ikehara, S., Muramatsu, S., Steinman, R.M.: Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176, 1693–1702 (1992). doi:10.1084/jem.176.6.1693
Sasaki, K., Kurata-Miura, K., Ujita, M., Angata, K., Nakagawa, S., Sekine, S., Nishi, T., Fukuda, M.: Expression cloning of cDNA encoding a human beta-1, 3-N-acetylglucosaminyltransferase that is essential for poly-N-acetyllactosamine synthesis. Proc. Natl. Acad. Sci. USA 94, 14294–14299 (1997). doi:10.1073/pnas.94.26.14294
Weinhold, B., Seidenfaden, R., Rockle, I., Muhlenhoff, M., Schertzinger, F., Conzelmann, S., Marth, J.D., Gerardy-Schahn, R., Hildebrandt, H.: Genetic ablation of polysialic acid causes severe neurodevelopmental defects rescued by deletion of the neural cell adhesion molecule. J. Biol. Chem. 280, 42971–42977 (2005). doi:10.1074/jbc.M511097200
Curreli, S., Arany, Z., Gerardy-Schahn, R., Mann, D., Stamatos, N.M.: Polysialylated neuropilin-2 is expressed on the surface of human dendritic cells and modulates dendritic cell-T lymphocyte interactions. J. Biol. Chem. 282, 30346–30356 (2007). doi:10.1074/jbc.M702965200
Kitagawa, H., Shimakawa, H., Sugahara, K.: The tumor suppressor EXT-like gene EXTL2 encodes an alpha1, 4-N-acetylhexosaminyltransferase that transfers N-acetylgalactosamine and N-acetylglucosamine to the common glycosaminoglycan-protein linkage region. The key enzyme for the chain initiation of heparan sulfate. J. Biol. Chem. 274, 13933–13937 (1999). doi:10.1074/jbc.274.20.13933
Wegrowski, Y., Milard, A.L., Kotlarz, G., Toulmonde, E., Maquart, F.X., Bernard, J.: Cell surface proteoglycan expression during maturation of human monocytes-derived dendritic cells and macrophages. Clin. Exp. Immunol. 144, 485–493 (2006). doi:10.1111/j.1365-2249.2006.03059.x
Jenner, J., Kerst, G., Handgretinger, R., Muller, I.: Increased alpha2, 6-sialylation of surface proteins on tolerogenic, immature dendritic cells and regulatory T cells. Exp. Hematol. 34, 1212–1218 (2006). doi:10.1016/j.exphem.2006.04.016
Martinez, F.O., Gordon, S., Locati, M., Mantovani, A.: Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 177, 7303–7311 (2006)
Suzuki, A., Yamakawa, M., Tsukamoto, M.: The adhesion molecules, l-selectin and sialyl lewis x, relate to the formation of the follicular dendritic cell-lymphocyte cluster in the mantle zone. Immunol. Lett. 79, 181–187 (2001). doi:10.1016/S0165-2478(01)00282-6
Crocker, P.R.: Siglecs in innate immunity. Curr. Opin. Pharmacol. 5, 431–437 (2005). doi:10.1016/j.coph.2005.03.003
Campbell, B.J., Yu, L.G., Rhodes, J.M.: Altered glycosylation in inflammatory bowel disease: a possible role in cancer development. Glycoconj. J. 18, 851–858 (2001). doi:10.1023/A:1022240107040
Coulouarn, C., Lefebvre, G., Derambure, C., Lequerre, T., Scotte, M., Francois, A., Cellier, D., Daveau, M., Salier, J.P.: Altered gene expression in acute systemic inflammation detected by complete coverage of the human liver transcriptome. Hepatology 39, 353–364 (2004). doi:10.1002/hep.20052
Van Dijk, W., Brinkman-Van der Linden, E.C., Havenaar, E.C.: Occurrence and possible function of inflammation-induced expression of sialyl Lewis-x on acute-phase proteins. Adv. Exp. Med. Biol 435, 145–150 (1998)
Kim, B.T., Kitagawa, H., Tanaka, J., Tamura, J., Sugahara, K.: In vitro heparan sulfate polymerization: crucial roles of core protein moieties of primer substrates in addition to the EXT1-EXT2 interaction. J. Biol. Chem. 278, 41618–41623 (2003). doi:10.1074/jbc.M304831200
Brigl, M., Bry, L., Kent, S.C., Gumperz, J.E., Brenner, M.B.: Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat. Immunol. 4, 1230–1237 (2003). doi:10.1038/ni1002
De Libero, G., Moran, A.P., Gober, H.J., Rossy, E., Shamshiev, A., Chelnokova, O., Mazorra, Z., Vendetti, S., Sacchi, A., Prendergast, M.M., Sansano, S., Tonevitsky, A., Landmann, R., Mori, L.: Bacterial infections promote T cell recognition of self-glycolipids. Immunity 22, 763–772 (2005). doi:10.1016/j.immuni.2005.04.013
Mattner, J., Debord, K.L., Ismail, N., Goff, R.D., Cantu 3rd, C., Zhou, D., Saint-Mezard, P., Wang, V., Gao, Y., Yin, N., Hoebe, K., Schneewind, O., Walker, D., Beutler, B., Teyton, L., Savage, P.B., Bendelac, A.: Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434, 525–529 (2005). doi:10.1038/nature03408
Paget, C., Mallevaey, T., Speak, A.O., Torres, D., Fontaine, J., Sheehan, K.C., Capron, M., Ryffel, B., Faveeuw, C., Leite de Moraes, M., Platt, F., Platt, F., Trottein, F., Trottein, F.: Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity 27, 597–609 (2007). doi:10.1016/j.immuni.2007.08.017
Salio, M., Speak, A.O., Shepherd, D., Polzella, P., Illarionov, P.A., Veerapen, N., Besra, G.S., Platt, F.M., Cerundolo, V.: Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc. Natl. Acad. Sci. USA 104, 20490–20495 (2007). doi:10.1073/pnas.0710145104
Tupin, E., Kinjo, Y., Kronenberg, M.: The unique role of natural killer T cells in the response to microorganisms. Nat. Rev. Microbiol. 5, 405–417 (2007). doi:10.1038/nrmicro1657
Garcia-Vallejo, J.J., Gringhuis, S.I., van Dijk, W., van Die, I.: Gene expression analysis of glycosylation-related genes by real-time polymerase chain reaction. Methods Mol. Biol. 347, 187–209 (2006)
Groux-Degroote, S., Krzewinski-Recchi, M.A., Cazet, A., Vincent, A., Lehoux, S., Lafitte, J.J., Van Seuningen, I., Delannoy, P.: IL-6 and IL-8 increase the expression of glycosyltransferases and sulfotransferases involved in the biosynthesis of sialylated and/or sulfated Lewisx epitopes in the human bronchial mucosa. Biochem. J. 410, 213–223 (2008). doi:10.1042/BJ20070958
Acknowledgments
We acknowledge Dr Juan J. Garcia-Vallejo (VU Medical Center, Amsterdam, The Netherlands) for the gift of some oligonucleotides used for the qPCR analysis. We also thank Pr. R. Gerardy-Schahn (Medizinische Hochschule, Hannover, Germany) for the gift of the anti-PSA Ab.
This work was supported by the Institut National de la Santé et de la Recherche Médicale, the Pasteur Institute of Lille, the University of Lille 2, the Contrat de Plan Etat Région 2000–2006 (CPER)/FEDER (Fonds Européen de Développement Régional) and l’Agence Nationale de la Recherche (ANR) (program MIE grant R08066ES/RPV08036ESA). This work was also supported by Consortium for Functional Glycomics Grant GM-62116 from the National Institutes of Health. CP was recipient of a doctoral fellowship from the Conseil Régional Nord—Pas-de-Calais / INSERM. FT is supported by the Centre National de la Recherche Scientifique, CF and PG by the INSERM and SGD, MAKR and PD by the University of Lille 1.
Conflict of interest statement
None declared.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Trottein, F., Schaffer, L., Ivanov, S. et al. Glycosyltransferase and sulfotransferase gene expression profiles in human monocytes, dendritic cells and macrophages. Glycoconj J 26, 1259–1274 (2009). https://doi.org/10.1007/s10719-009-9244-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-009-9244-y