Abstract
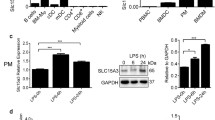
Macrophages are known to express various types of endocytosis receptors that mediate the removal of foreign pathogens. Macrophage asialoglycoprotein-binding protein (M-ASGP-BP) is a Gal/GalNAc-specific lectin, which functions as an endocytosis receptor. We found here that LPS is able to down-regulate the mRNA expression of M-ASGP-BP in a time-dependent manner using thioglycolate-elicited rat and mouse peritoneal macrophages. However, LPS does not modulate the mRNA expression of M-ASGP-BP from macrophages of C3H/HeN mice, which have a point mutation of TLR4, the primary LPS receptor. Furthermore, an inhibitor of NF-κB was observed to efficiently block the suppressive effect of LPS on M-ASGP-BP as well as to inhibit the phosphorylated IκB. These results demonstrate that the mRNA expression of M-ASGP-BP is down-regulated by the LPS-mediated TLR4 pathway involving NF-κB activation, suggesting that engagement of M-ASGP-BP by LPS may yield a negative signal that interferes with the LPS-induced positive signals mediated by proinflammatory cytokines.





Similar content being viewed by others
Abbreviations
- M-ASGP-BP:
-
macrophage asialoglycoprotein-binding protein
- MGL:
-
macrophage C-type galactose/N-acetylgalactosamine-specific lectin
- Mϕ:
-
macrophage
- LPS:
-
lipopolysaccharide
- TLR4:
-
toll-like receptor 4
- MAPK:
-
mitogen-activated protein kinase
- ERK:
-
extracellular signal-regulated protein kinase
- JNK:
-
c-Jun amino-terminal kinase
- NF-κB:
-
nuclear factor kappa B
- IκB:
-
inhibitor of NF-κB
References
Lewis, C., McGee J.O.D.: The Macrophage: The Natural Immune System. IRL at Oxford University Press, Oxford (1992)
Wileman, T.E., Lennartz, M.R., Stahl, P.D.: Identification of the macrophage mannose receptor as a 175-kDa membrane protein. Proc. Natl. Acad. Sci. U.S.A. 83, 2501–2505 (1986)
Drickamer. K., Taylor, M.E.: Biology of animal lectins. Annu. Rev. Cell Biol. 9, 237–264 (1993)
Guha, M., Mackman, N.: LPS induction of gene expression in human monocytes. Cell. Signal. 13, 85–94 (2001)
Lynn, W.A., Cohen, J.: Adjunctive therapy for septic shock: a review of experimental approaches. Clin. Infect. Dis. 20, 143–158 (1995)
Williams, K.J., Tabas, I.: The response-to-retention hypothesis of atherogenesis reinforced. Curr. Opin. Lipidol. 9, 471–474 (1998)
Ii, M., Kurada, H., Itoh, N., Yamashina, I., Kawasaki, T.: Molecular cloning and sequence analysis of cDNA encoding the macrophage lectin specific for galactose and N-acetylgalactosamine. J. Biol. Chem. 265, 11295–11298 (1990)
Ozaki, K., Ii, M., Itoh, N., Kawasaki, T.: Expression of a functional asialoglycoprotein receptor through transfection of a cloned cDNA that encodes a macrophage lectin. J. Biol. Chem. 267, 9229–9235 (1992)
Sato, M., Kawakami, K., Osawa, T., Toyoshima, S.: Molecular cloning and expression of cDNA encoding a galactose/N-acetylgalactosamine-specific lectin on mouse tumoricidal macrophages. J. Biochem. 111, 331–336 (1992)
Russell, M.E., Utans, U., Wallace, A.F., Liang, P., Arceci, R.J., Karnovsky M.J., Wyner, L.R., Yamashida, Y., Tarn, C.: Identification and upregulation of galactose/N-acetylgalactosamine macrophage lectin in rat cardiac allografts with arteriosclerosis. J. Clin. Invest. 94, 722–730 (1994)
Katsuyama, R., Morioka, A., Oka, S., Kawasaki, T.: Expression of macrophage asialoglycoprotein-binding protein is induced through MAPK classical pathway. Biochem. Biophys. Res. Commun. 280, 1269–1273 (2001)
Mikolajczak, S.A., Ma, B.Y., Yoshida, T., Yoshida, R., Kelvin, D.J., Ochi, A.: The modulation of CD40 ligand signaling by transmembrane CD28 splice variant in human T cells. J. Exp. Med. 199, 1025–1031 (2004)
Poltorak, A., He, X., Smirnova, I., Liu, M., Van Huffel, C., Du, X., Birdwell, D., Alejos, E., Silva, M., Galanos, C., Freudenberg, M., Castagnoli, P.R., Layton, B., Beutler, B.: Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in TLR4 gene. Science 282, 2085–2088 (1998)
Herrera-Velit, P., Reiner, N.E.: Bacterial lipopolysaccharide induces the association and coordinate activation of p53/56lyn and phosphatidylinositol 3-kinase in human monocytes. J. Immunol. 156, 1157–1165 (1996)
Han, J., Lee, J.D., Bibbs, L., Ulevitch, R.J.,:A MAP kinase targeted by endotoxin and hyposmolarity in mammalian cells. Science 265, 808–811 (1994)
Jakway, J.P., DeFranco, A.L.: Pertussis toxin inhibition of B cell and macrophage response to bacterial lipopolysaccharide. Science 234, 743–746 (1986)
Sanghera, J.S, Weinstein, S.L., Aluwalia, M., Gien, J., Pelech, S.L.: Activation of multiple proline-directed kinases by bacterial lipopolysaccharide in murine macrophages. J. Immunol. 156, 4457–4465 (1996)
Sato, K., Imai, Y., Irimura, T.: Contribution of dermal macrophage trafficking in the sensitization phase of contact hypersensitivity. J. Immunol. 161, 6835–6844 (1998)
Chun, K.H., Imai, Y., Higashi, N., Irimura, T.: Migration of dermal cells expressing a macrophage C-type lectin during the sensitization phase of delayed-type hypersensitivity. J. Leukoc. Biol. 68, 471–478 (2000)
Guha, M., Mackman, N.: LPS induction of gene expression in human monocytes. Cell. Signal. 13, 85–94 (2001)
Swantek, J.L., Christerson, L., Cobb, M.H.: Lipopolysaccharide-induced tumor necrosis factor-α promoter activity is inhibitor of nuclear factor-κB kinase-dependent. J. Biol. Chem. 274, 11667–11671 (1999)
Higashi, N., Fujioka, K., Denda-Nagai, K., Hashimoto, S., Nagai, S., Sato, T., Fujita, Y., Morikawa, A., Tsuiji, M., Miyata-Takeuchi, M., Sano, Y., Suzuki, N., Yamamoto, K., Matsushima, K., Irimura, T.: The macrophage C-type lectin specific for galactose/N-acetylgalactosamine is an endocytic receptor expressed on monocyte-derived immature dendritic cell. J. Biol. Chem. 277, 20686–20693 (2002)
Tsuiji, M., Fujimori, M., Ohashi, Y., Higashi, N., Onami, T.M., Hedrick, S.M., Irimura, T.:Molecular cloning and characterization of a novel mouse macrophage C-type lectin, mMGL2, which has a distinct carbohydrate specificity from Mmgl. J. Biol. Chem. 277, 28892–28901 (2002)
Shepherd, V.L., Abdolrasulnia, R., Garrett, M., Cowan, H.B.: Down-regulation of mannose receptor activity in macrophages after treatment with lipopolysaccharide and phorbol esters. J. Immunol. 145, 1530–1536 (1990)
Van Lenten, B.J., Fogelman, A.M., Seager, J., Ribi, E., Haberland, M.E., Edwards, P.A.: Bacterial endotoxin selectively prevents the expression of scavenger-receptor activity on human monocyte-macrophages. J. Immunol. 134, 3718–3721 (1985)
Weiel, J.E., Adams, D.O., Hamilton, T.A.: Biochemical models of gamma-interferon action: Altered expression of transferrin receptors on murine peritoneal macrophages after treatment in vitro with PMA or A23187. J. Immunol. 134, 293–298 (1985)
Pahl, H.: Activators and target genes of Rel/NF-κB transcription factors. Oncogene 18, 6853–6866 (1999)
Ma, W., Lim, W., Gee, K., Aucoin, S., Nandan, D., Kozlowski, M., Diaz-Mitoma, F., Kumar, A.: The p38 mitogen activated kinase pathway regulates the human IL-10 promoter via the activation of Sp1 transcription factor in lipopolysaccharide-stimulated human macrophages. J. Biol. Chem. 276, 13664–13674 (2001)
Hambleton, J., Weinstein, S.L., Lem, L., DeFranco, A.L.: Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc. Natl. Acad. Sci. U.S.A. 93, 2774–2778 (1996)
Groupp, E.R., Donovan-Peluso, M.: Lipopolysaccharide induction of THP-1 cells activates binding of c-Jun, Ets, Egr-1 to the tissue factor promoter. J. Biol. Chem. 271, 12423–12430 (1996)
Acknowledgments
The authors would like to thank Ms. Tomoko Tominaga for the secretarial assistance.
This work was supported in part by a Grant-in-Aid for Scientific Research on Priority Areas, A-14082203, to T. Kawasaki, and for Scientific Research, C-18590471, to B.Y. Ma from the Japan Society for the Promotion of Science, Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ma, B.Y., Kaihama, M., Nonaka, M. et al. LPS suppresses expression of asialoglycoprotein-binding protein through TLR4 in thioglycolate-elicited peritoneal macrophages. Glycoconj J 24, 243–249 (2007). https://doi.org/10.1007/s10719-007-9031-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-007-9031-6