Abstract
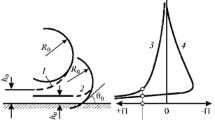
An apparatus for determining the wettability of quartz sand was developed. It was shown that as the temperature increases, the surface wettability of the minerals present in the glass quartz sand increases owing to a reduction of the slippage of the liquid along the solid surface and, in consequence, the aggregative stability of their suspensions increases.






Similar content being viewed by others
References
O. A. Slyusar and V. M. Uvarov, “Wetting of solid surfaces by solutions of modifying additives,” Steklo Keram., No. 4, 36 – 38 (2014); O. A. Slyusar and V. M. Uvarov, “Wetting of solid surfaces by solutions of modifying additives,” Glass Ceram., 71(3 – 4), 140 – 142 (2014).
V. A. Pchelin, V. Ya. Yampol’skii, V. V. Yaminskii, and V. V. Ievleva, “On the modification of water near hydrophobic surfaces,” in: Reports at the 5th Conference on the Surface Forces: Surfaces Forces in Thin Films and Stability of Colloids [in Russian], Moscow (1972), Nauka, Moscow (1974), pp. 43 – 50.
S. I. Evdokimov and A. M. Pan’shin, “Laws of contact interactions between the particles in a polydisperse mineral system,” Izv. Vyssh. Uchebn. Zaved., Tsvetn. Metall., No. 6, 4 – 19 (2007).
O. I. Vinogradova, Particularities of Hydrodynamic and Equilibrium Interactions of Hydrophobic Surfaces, Author’s Abstract of Doctoral’s Thesis [in Russian], Institute of Physical Chemistry, Russian Academy of Sciences, Moscow (2000).
N. V. Churaev, “Surface forces and physical chemistry of surface phenomena,” Usp. Khim., 73(11), 26 – 38 (2004).
N. B. Ur’ev, “Physical-chemical dynamics of disperse systems,” Usp. Khim., 73(1), 39 – 62 (2004).
N. S. Nerpina, “Flow of polar liquids with hydrogen bonds through capillaries with diophylic walls,” in: Reports at the 5th Conference on Surface Forces: Surface Forces in Thin Films and Stability of Colloids [in Russian], Nauka, Moscow (1972), pp. 76 – 79.
A. C. Simonsen, P. L. Hansen and B. Klosgen, “Nanobubbles give evidence of incomplete wetting at a hydrophobic interface,” J. Colloid Interface Sci., 273, 291 – 299 (2004).
M. A. Hampton and A. V. Nguyen, “Nanobubbles and the nanobubble bridging capillary force,” Adv. Colloid Interface Sci., 154(1 – 2), 30 – 55 (2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Steklo i Keramika, No. 9, pp. 20 – 23, September, 2015.
Rights and permissions
About this article
Cite this article
Evdokimov, S.I., Galachieva, S.V., Puzin, V.S. et al. Development and Investigation of an Apparatus for Determining the Wetting of Quartz Sand. Glass Ceram 72, 323–326 (2016). https://doi.org/10.1007/s10717-016-9783-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10717-016-9783-1