The development of a reliable system for protecting beryllium from oxidation and the sublimation of its toxic oxides during heating at high temperatures is examined. The results of investigations of the oxidation kinetics of beryllium with no coating, with a passive film, and with a composite coating at heating temperatures to 900°C in 50 h are presented. It is shown that a composite coating consisting of a passivating layer and a heat-resistant coating based on the system SiO2–B2O3–R2O is effective.
Similar content being viewed by others
Possessing a combination of extra-ordinary physical properties (low density, record high specific toughness, relatively high thermal conductivity and thermal diffusivity) beryllium holds promise for application in rocket-space, aviation, and nuclear technologies [1, 2].
The complex conditions under which beryllium operating operate make it necessary to develop new protective coatings satisfying specific requirements developed for modern articles.
At high temperatures beryllium is subject to surface oxidation with toxic beryllium compounds being released into the atmosphere.
It can be concluded on the basis of the very high heat and free energy of interaction of beryllium with oxygen that the oxygen affinity of beryllium is high and therefore the interaction between them is strong.
According to Wagner’s theory oxidation follows the law
where Δm is the mass gain of the samples over time τ and k is the oxidation rate constant.
When beryllium interacts with gaseous substances, which is accompanied by the formation of a non-protective oxide film, the interaction passes through the following successive stages:
-
transport of gaseous substances to the phase interface;
-
adsorption of gas on the surface of the metal;
-
chemical interaction;
-
removal (desorption, sublimation) of the products of corrosion from the interaction zone.
For n ≤ 1 the oxide film is not protective. In this case the slowest (and limiting) stage of the process is chemical interaction. The growth rate of the film will be constant:
where y is the thickness of the film, τ is the oxidation time of the metal, and k is the rate constant of the chemical reaction.
Integration yields
Thus, the law of oxidation is linear.
When a protective film forms on a beryllium surface a stage where oxygen diffuses through the film is added and the rate of the process is determined by rate of this stage. As temperature increases the rate of the chemical reaction increases rapidly and diffusion increases very slowly, as a result of which at high temperatures the corrosion rate starts to follow the laws of diffusion.
The stronger the protective properties of the film, the slower its growth rate dy/dτ = ky is. Integration gives a parabolic relation y 2 = 2kτ + const, const → 0, and y 2 = 2kτ.
Experiments confirm the dependences obtained for the oxidizability of beryllium. The samples were observed to undergo two stages of oxidation with each stage having its own mechanical interactions:
-
1)
induction — the rate of the chemical reaction leading to oxidation of the metal determines the growth of a porous film;
-
2)
diffusion — the ion motion in the interstitial space of a crystal lattice determines film growth.
Oxidation during the induction period (1 h) follows a linear law (unprotected oxidation). In addition, the limits of the induction period narrow as temperature increases. The oxidation mechanism at the second stage includes the ion diffusion on the one hand and the formation of microcracks, which accelerate the reaction, on the other. Cracks result from high stresses, which increase as the oxide film grows. This is why the oxide film losses its protective properties. Intense crack formation enables oxygen to reach fresh surfaces in the metal, which rapidly increases the interaction rate.
An important task of the present studies is to develop a reliable system to protect beryllium from oxidation and sublimation of toxic beryllium oxides when heated to high temperatures [3].
The use of a complex protective system consisting of a passivating film and inorganic high-temperature heat-resistant coatings is the most effective protection from oxidation and sublimation of toxic beryllium compounds [4]. The development of a complex protective system is an extremely difficult problem, because two coatings with different chemical composition and technological deposition and formation parameters must be combined.
The main purpose of the passivating layer is to protect beryllium from oxidation at the initial stages of oxidation (400 – 600°C), develop an intermediate layer with strong bonding between the beryllium and the layer and increase the stability of the heat-resistant coating at high temperatures.
The principal requirements for the coatings were formulated on the basis of the applications, operating conditions and functions of the coatings:
-
adjustment of chemical processes occurring at the interfaces metal – passivating coating – heat-resistant coating;
-
careful preparation of the sample surface under chemical passivation (chemical or electrochemical ionization of the surface);
-
heat treatment of the passivating layer is mandatory in order to increase the protective properties of the passive film and secure high-quality bonding at the metal – passivating coating – heat-resistance coating;
-
the formation temperature of a heat-resistant coating must be less than the heat-treatment temperature of the passivating layer.
A number of requirements are imposed on the solutions used for surface passivation:
-
the metal losses occurring during the removal of contaminants from the surface of the samples must be minimized;
-
the surface must not dissolve;
-
sludge must not form on the surface during etching.
Chromatization in a solution consisting of a mixture of potassium chromate and hydrofluoric acid satisfies all these requirements.
The composition of the solution for passivating beryllium must ensure that a redox-reaction occurs and that the compounds from which a coating is formed are produced. This requires on the metal – solution boundary a definite oxidizer concentration corresponding to the pH and a definite ratio of the cations and anions which are capable of forming the insoluble matter of the coating. As the chromatizing solution interacts with beryllium, beryllium ions and trivalent chromium ions accumulate and the solution pH on the metal – solution boundary becomes more alkaline, which creates conditions for the formation of the insoluble matter of the coating and precipitation of this matter on the surface of the metal. The time dependence of the formation and growth of a coating on beryllium in a solution containing potassium bichromate and hydrofluoric acid is parabolic.
To increase the heat-tolerance of the passivating layer and secure high-quality bonding with the heat-resistant layer the effect of heat-treatment (annealing) on the heat-resistance of the layer was studied.
Figure 1 displays data on the oxidation kinetics of beryllium samples with a passive film obtained by the optimal technology and subjected to additional heat treatment in the temperature interval 600 – 800°C. Beryllium samples with different surface states were tested on a continual weighing apparatus for oxidizability.
The protective action of the passive film is more effective after heat treatment.
The studies performed show that the optimal heat-treatment temperature for the passive film is 600°C. Beryllium samples with a passive film heat-treated at 600°C have smaller mass increases over the entire 5 h of the tests — 0.05 mg/cm2, while for samples with an unannealed passive film the mass increase is 0.4 mg/cm2, i.e., 8 times larger.
X-ray phase analysis in combination with spectral microanalysis showed that after firing at 600°C a passive film contains chromium compounds over the entire surface. The following compounds were determined: Cr(OH)3, Be(CrO2)2, BeO, Cr2O3 and Be(OH)2. The film obtained is bright green. Thus, the surface of the beryllium samples was coated with a film with a non-uniform chemical composition. Chromium compounds and beryllium oxide predominate in the film. When the heat-treatment temperature of the passive film is increased to 800°C the film becomes more open and its protective properties and the quality of the bonding with beryllium decrease.
The experimental data show that additional annealing increases the protective properties of the film. This is due to a decrease of the porosity of the film and formation of thermodynamically stable beryllium and chromium compounds.
The following factors must be taken into account when choosing the directions of synthesis of protective heat-resistant coatings for beryllium alloys:
-
the coating must be formed at a temperature lower than the oxidation onset temperature for beryllium, i.e., no higher than 500°C;
-
the coating slip must be workable and give a uniform distribution of the coating over the entire surface of the passivating layer of the sample;
-
the components of the coating must be temperature stable and ensure a continuous, high degree of protection of the metal surface at high temperatures and with long standing times.
The protective heat-resistant coatings for beryllium were synthesized in the systems SiO2–B2O3–R2O and SiO2–Al2O3–R2O.
To slow down the oxidation of beryllium substances with high entropy of oxygen vacancies were introduced into the protective coatings: the oxides Al2O3 and Cr2O3. Aluminum and chromium oxides as well as silicon dioxide are distinguished by low electric conductivity, which in conformance to Wagner’s theory ensures a low rate of diffusion in the oxides. In the case where a continuous layer is formed when these materials melt they limit oxygen diffusion to the surface of the metal.
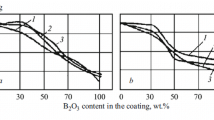
The main physical-chemical properties of the fused frits and technological properties of the experimental slips are given in Tables 1 and 2.
The data obtained show that the deformation onset temperature of the synthesized frits is lower than the oxidation onset temperature of beryllium; the linear thermal expansion coefficients (CLTE) are close to those of beryllium, which will make it possible to protect beryllium at the early stages of heating and will ensure bonding of the coating with the substrate during prolonged operation of components.
The technological properties of the protective-coating slips based on fused frits and modifying fillers are stable.
As the heating temperature and aging time increase, the protective effect of the composite coating increases. This is confirmed by the comparative results of thermogravimetric tests performed on beryllium samples with and without a coating (Fig. 2).
Spectral analysis and laser microanalysis show that interaction processes in which Cr, Be, and Si compounds are formed occur between beryllium, a passive film, and an enamel coating. It was found that Cr and Be are present on the inner surface and Cr on the outer surface of a coating.
Experimental studies have determined the following:
-
the optimal composition of the composite protective system and the temperature – time regime for its formation;
-
a passivating film with additional heat-treatment at 600°C and protective heat-resistant coating of the system SiO2–B2O3–R2O with Cr2O3 as the modifying filler;
-
the formation regime for the protective coating 560 – 650°C.
The enhanced protective properties of the new coating with low formation temperature can be explained by the fact that during annealing beryllium and chromium diffuse into the coating and form compounds which partially dissolve in the protective layer of the coating, making it more refractory and inert. In addition, at the initial stage of heating the coating permeates the open passive film and fills the numerous cracks on the passive film, thereby increasing its density. Composite protective coatings on beryllium slow down corrosion processes sharply, impede the diffusion of aggressive components from a gaseous medium and impede chemical reactions at the boundary oxidative medium – coating – passive film – metal at high temperatures.
The results of sampling air for the content of beryllium compounds over the entire process heating cycle have shown that no toxic beryllium compounds are present in air.
These studies have shown that the composite protective coating developed gives high-quality protection of beryllium against oxidation and sublimation of toxic beryllium compounds at high temperatures and during prolonged heating.
References
Beryllium: Science and Technology [Russian translation], Metallurgiya, Moscow (1984).
I. N. Fridlyander and K. P. Yatsenko, “Beryllium alloys — promising direction of aerospace materials engineering,” in: Aircraft Materials and NTS Technology [in Russian], Moscow (2000), pp.3–6.
S. S. Solntsev, Protective Technological Coatings and Refractory Enamels [in Russian], Mashinostroenie, Moscow (1984), pp.12 –29.
S. S. Solntsev and V. A. Rozenenkova, “Protective technological coatings,” Tekh. Tekhnol. Silikatov,No.1–2,23–33 (2005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Steklo i Keramika, No. 4, pp. 12 – 15, April, 2012.
Rights and permissions
About this article
Cite this article
Kablov, E.N., Solntsev, S.S., Rozenenkova, V.A. et al. Composite glass-metal coatings for protecting beryllium at high temperatures. Glass Ceram 69, 113–116 (2012). https://doi.org/10.1007/s10717-012-9426-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10717-012-9426-0